Freestanding transparent metallic network based ultrathin, foldable and designable supercapacitors†
Abstract
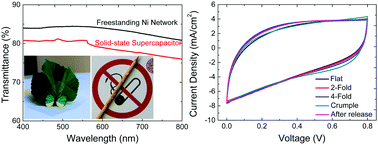
Fully integrated ultrathin, transparent and foldable energy storage devices are essential for the development of smart wearable electronics, yet typical supercapacitor electrodes are substrate-supported which limits their thickness, transparency and mechanical properties. Employing freestanding transparent electrodes with no substrate support could bring ultrathin, foldable and designable supercapacitors closer to reality. Herein, we report a freestanding, ultrathin (<5 μm), highly conductive (3 × 104 S cm−1), highly transparent (>84% transmittance) and foldable metallic network electrode, loaded with MnO2 by electrochemical deposition, as a supercapacitor electrode. The freestanding metallic network electrode is fabricated via a simple and low-cost laser direct-writing micro-patterning technique followed by a selective electrodeposition process, where the metallic network patterns, network periods, metal thickness and also the electrode film patterns can be designed for different applications. The obtained freestanding MnO2@Ni network electrode delivers an outstanding areal capacitance of 80.7 mF cm−2 and long-term performance stability (96.3% after 10 000 cycles). Moreover, the symmetric solid-state supercapacitors employing the freestanding MnO2@Ni network electrode not only show high areal capacitance as well as high optical transparency (>80% transmittance), but also can be tailored, attached, folded, rolled up, and crumpled into any object or various shapes with only slight performance degradation. The advent of such freestanding transparent metallic network electrodes may open up a new avenue for realizing fully integrated ultrathin, foldable and designable supercapacitors towards self-powered wearable electronics.
- This article is part of the themed collection: 2017 Energy and Environmental Science HOT articles


 Please wait while we load your content...
Please wait while we load your content...