Flexible/shape-versatile, bipolar all-solid-state lithium-ion batteries prepared by multistage printing†
Abstract
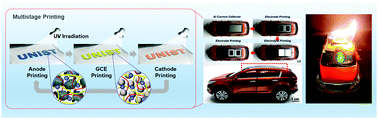
Bipolar all-solid-state lithium-ion batteries (LIBs) have attracted considerable attention as a promising approach to address the ever-increasing demand for high energy and safety. However, the use of (sulfide- or oxide-based) inorganic solid electrolytes, which have been the most extensively investigated electrolytes in LIBs, causes problems with respect to mechanical flexibility and form factors in addition to their longstanding issues such as chemical/electrochemical instability, interfacial contact resistance and manufacturing processability. Here, we develop a new class of flexible/shape-versatile bipolar all-solid-state LIBs via ultraviolet (UV) curing-assisted multistage printing, which does not require the high-pressure/high-temperature sintering processes adopted for typical inorganic electrolyte-based all-solid-state LIBs. Instead of inorganic electrolytes, a flexible/nonflammable gel electrolyte consisting of a sebaconitrile-based electrolyte and a semi-interpenetrating polymer network skeleton is used as a core element in the printed electrodes and gel composite electrolytes (GCEs, acting as an ion-conducting separator membrane). Rheology tuning (toward thixotropic fluid behavior) of the electrode and GCE pastes, in conjunction with solvent-drying-free multistage printing, enables the monolithic integration of in-series/in-plane bipolar-stacked cells onto complex-shaped objects. Because of the aforementioned material and process novelties, the printed bipolar LIBs show exceptional flexibility, form factors, charge/discharge behavior and abuse tolerance (nonflammability) that far exceed those achievable with inorganic-electrolyte-based conventional bipolar cell technologies.


 Please wait while we load your content...
Please wait while we load your content...