Open Access Article
Open Access ArticleBiomass-based chemical looping technologies: the good, the bad and the future
Xiao
Zhao†
 ab,
Hui
Zhou†
ab,
Hui
Zhou†
 cd,
Vineet Singh
Sikarwar
ab,
Ming
Zhao
cd,
Vineet Singh
Sikarwar
ab,
Ming
Zhao
 *ab,
Ah-Hyung A.
Park
*ab,
Ah-Hyung A.
Park
 *cd,
Paul S.
Fennell
*cd,
Paul S.
Fennell
 e,
Laihong
Shen
f and
Liang-Shih
Fan
e,
Laihong
Shen
f and
Liang-Shih
Fan
 g
g
aSchool of Environment, Tsinghua University, Beijing 100084, China. E-mail: ming.zhao@tsinghua.edu.cn; Tel: +86 10 62784701
bKey Laboratory for Solid Waste Management and Environment Safety, Ministry of Education of China, Tsinghua University, Beijing 100084, China
cDepartment of Chemical Engineering, Columbia University, New York, NY 10027, USA. E-mail: ap2622@columbia.edu
dLenfest Center for Sustainable Energy, Columbia University, New York, NY 10027, USA
eDepartment of Chemical Engineering, Imperial College London, South Kensington, London, SW7 2AZ, UK
fKey Laboratory of Energy Thermal Conversion and Control of Ministry of Education, School of Energy and Environment, Southeast University, Nanjing, China
gWilliam G. Lowrie Department of Chemical and Biomolecular Engineering, The Ohio State University, Columbus, OH 43210, USA
First published on 19th May 2017
Abstract
Biomass is a promising renewable energy resource despite its low energy density, high moisture content and complex ash components. The use of biomass in energy production is considered to be approximately carbon neutral, and if it is combined with carbon capture technology, the overall energy conversion may even be negative in terms of net CO2 emission, which is known as BECCS (bioenergy with carbon capture and storage). The initial development of BECCS technologies often proposes the installation of a CO2 capture unit downstream of the conventional thermochemical conversion processes, which comprise combustion, pyrolysis or gasification. Although these approaches would benefit from the adaptation of already well developed energy conversion processes and CO2 capture technologies, they are limited in terms of materials and energy integration as well as systems engineering, which could lead to truly disruptive technologies for BECCS. Recently, a new generation of transformative energy conversion technologies including chemical looping have been developed. In particular, chemical looping employs solid looping materials and it uniquely allows inherent capture of CO2 during the conversion of fuels. In this review, the benefits, challenges, and prospects of biomass-based chemical looping technologies in various configurations have been discussed in-depth to provide important insight into the development of innovative BECCS technologies based on chemical looping.
Broader contextSince carbon in biomass is obtained from atmospheric CO2, biomass is considered to be a carbon-neutral fuel. By circulating carbon to and from the biosphere through energy generation systems in the form of biomass, issues including climate change can be addressed. Other renewable energy options such as solar and wind can provide carbon-free electricity or even H2 by splitting water, and thus, they have been rapidly deployed to decarbonise the power sector. However, solar or wind energy cannot directly be used to produce carbon-based fuels and chemicals without additional carbon sources. Considering the ever-increasing need for carbon-based materials (both high density fuels and various chemicals), biomass utilisation is very important towards to allow a decarbonised sustainable future. Chemical looping processes (CLPs) have the advantage that CO2 is separated in situ, thus eliminating the need for an energy intensive CO2 capture unit. The direct use of solid fuels including biomass in CLPs has a number of difficulties including solid handling and separation challenges, tar formation, and deactivation of looping materials. However, biomass-based chemical looping processes (BCLPs) are important technologies for the future because they start with carbon-neutral fuels. Combined with appropriate CO2 storage options, BCLPs can allow net negative emissions of carbon. |
1. Introduction
The reduction of greenhouse gas (GHG) emissions is one of current society's greatest global challenges. There is a consensus that CO2 plays a key role in global warming,1,2 where the combustion of fossil fuels (coal, petroleum and natural gas) is the greatest contributor to CO2 emissions.3,4 In December, 2015 at the 21st Conference of the Parties of the United Nations Framework Convention on Climate Change (UNFCCC) in Paris, 147 countries set a global aspiration to limit the temperature increase to 1.5 °C above pre-industrial levels.5 With the Paris Agreement as a foundation, the world is searching for clean, renewable energy solutions to deal with GHG emissions. Moreover, economic benefits can be expected to be derived from renewable energy resource utilisation in the future, if a greenhouse gas emission trading scheme is established globally. Carbon capture and storage (CCS) is a necessary and viable pathway to reduce global CO2 emissions and to allow more sustainable use of fossil fuels.6,7Biomass has a long history as a major energy source and is considered to be an approximately carbon neutral renewable and abundant energy resource. CO2 is removed from the atmosphere, and solar energy is stored by biomass formation through photosynthesis. This means that using biomass as a fuel does not increase the total atmospheric CO2 inventory, and when combined with CCS, the overall system becomes carbon negative. Substituting biomass also lessens fossil fuel dependence. Based on the 2011 U.S. Billion-Ton Update, biomass is expected to replace 30% or more of the U.S. petroleum consumption by 2030.8 The European Union has outlined the National Renewable Energy Action Plan, which requires utilisation of up to 40% biomass for electricity, heating and cooling by 2020.9 In addition, as argued by the Intergovernmental Panel on Climate Change (IPCC) in its Fifth Assessment Report (AR5, 2013),10 bioenergy integrated with carbon capture and storage (BECCS) is expected to make a significant contribution to the sustainability of the biomass energy supply.
Modern biomass conversion technologies can typically be classified into biochemical or thermochemical processes. Thus far, biochemical processes are mainly constrained by their low energy efficiency, high water requirement, stringent feedstock property requirements and long conversion times.11–13 Conventional thermochemical conversion approaches, including combustion, gasification, and pyrolysis, developed for fossil fuels, can now use biomass feedstocks. Biomass combustion, pyrolysis, and gasification suffer from low efficiency mainly due to the relatively low energy density and high moisture content of biomass.
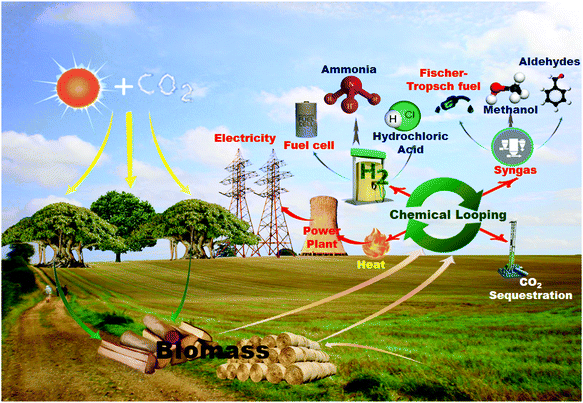
Chemical looping processes (CLPs) are relatively new and have potential in combustion, gasification and reforming of biomass. CLPs refer to the cyclic processes carried out as a set of subreactions using looping materials (LMs). During the reaction and regeneration of LMs, reduction–oxidation or carbonation–calcination cycles occur to produce a combination of heat, electricity, fuels and chemicals, as shown in Fig. 1.14–16 The concept of chemical looping dates back to the early 1900s for H2 production using steam-iron reforming.17 The principles were patented in 1946 for syngas production.18 The term chemical looping was first introduced in published literature in 1987 by Ishida et al.19 This concept has received intensive application and development since 1997.20
Biomass is an alternative fuel for CLPs, the product of which can be energy/heat or syngas. Additionally, the carbon neutrality of biomass can give more carbon credit for this technology and consequently economic advantages. In the U.S. Department of Energy's road map,14 CLPs provide the best cost reduction benefit among the various current and envisioned future technologies of CCS in a carbon-constrained scenario. In 2011, the UK's Energy Technologies Institute commissioned the TESBIC (Techno-economic Study of Biomass to Power with Integrated CO2 Capture).21 This report was completed in 2012, but the results were embargoed for a few years. The study compared 28 different potential combinations of CCS technologies (solvent scrubbing, oxyfuel, Integrated Gasification Combined Cycle (IGCC) and other advanced technologies including chemical looping). The study was unique, in that it combined consultants, industrial, and academic partners to conduct the investigation. Chemical looping was found to be a highly competitive technology, with the lowest potential cost of CO2 reduction among the technologies considered.
Herein, we review the field of biomass-based CLPs (BCLPs) and its promise to enhance conversion efficiency. Recent publications concerning biomass composition22–24 are also reviewed to assist in understanding the behaviour and fate of biomass in CLPs. We then summarise the merits and shortfalls for biomass as a fuel and thoroughly compare the conversion strategies. Previous review articles about CLPs mostly focused on the looping materials,16,25–27 operational experience,28,29 and/or process design.6,20 However, as an emerging significant focus of recent research,11,12,15 BCLPs have not been reviewed systematically. Therefore, this article critically approaches the terminologies and results, and concludes by discussing the advantages and disadvantages of the BCLPs technology and how its challenges might be addressed for long-term efficient and sustainable applications.
2. Biomass as a sustainable fuel
2.1. Biomass characteristics as a fuel and its thermo-physical properties
The definition of biomass has been intensely debated for many years. Broadly, biomass refers to any organic matter available on a renewable basis, including agricultural crops, agricultural waste and residue, wood and wood waste and residue, animal waste, municipal waste, and aquatic plants.11,30,31 Gases and liquids recovered from non-fossilised organics are also considered as biomass.30 Primary biomass comes directly from plants, animals or aquatic algae. Secondary biomass is waste produced from primary biomass. Agricultural solid waste, forestry residue, or industrial waste are important sources of biomass waste, along with municipal solid waste such as food scraps, woody waste and paper. Sewage sludge is also biomass since it contains a high proportion of organic matter derived from human excreta, grease and food waste.30 The use of biomass waste therefore not only allows a renewable approach for energy, but is also a potential strategy for recycling municipal solid waste.The utilisation of biomass is not a new concept. Before the 20th century woody biomass was the major energy source globally.30 However, the low energy density of biomass limited its large-scale applications, especially after fossil fuel use was industrialised. Since fossil fuels are a non-renewable energy resource that contributes to GHG emissions, biomass stands out again as a promising substitute. Biomass is the most abundant solid renewable resource with a global production of up to about 60 EJ per year.32 Woody biomass is still the most commonly used form and it has been estimated that energy derived from wood and woody waste accounts for almost 64% of the total biomass energy.33
Biomass is a complex mixture of organic and inorganic materials. The main components of organic materials are extractives and fibre or cell wall components, whereas the inorganic material is comprised of ash. Lignocellulosic biomass is believed to be the most promising fuel feedstock and its major constituents are polymeric carbohydrates.34 Although the polymeric compositions of biomass vary widely, they are usually comprised of three major constituents: cellulose (42–49 wt%), hemicellulose (16–23 wt%) and lignin (21–39 wt%).35,36 Cellulose is the skeletal structure of biomass which has the generic formula (C6H10O5)n. It is highly polymerised into glucan chains and its inherent bond is glycosidic linkages.37 Hemicellulose is shorter-chained and more amorphous which make it partially soluble in water, whereas lignin is a complex highly branched polymer that holds the cellulose fibres together to provide the mechanical strength of the cell wall.30
Classification of a solid fuel according to its atomic ratio allows correlation of its energy density and heating values. Based on the Van Krevelen diagram for various fuels (Fig. 2a), biomass has relatively higher molar ratios of H/C (1.2–1.8) and O/C (0.4–0.9) among all hydrocarbon fuels, whereas for coal, its H/C molar ratio ranges from 0.3–1.0 and O/C ranges from 0–0.25. The combustion of biomass with high contents of H and O causes high volatile and liquid yields thereby reducing its overall energy conversion efficiency. In addition, fuels with higher H/C ratios have a greater heat of combustion, whereas fuels with higher O/C ratios have higher CO2 emission per amount of energy release.38,39
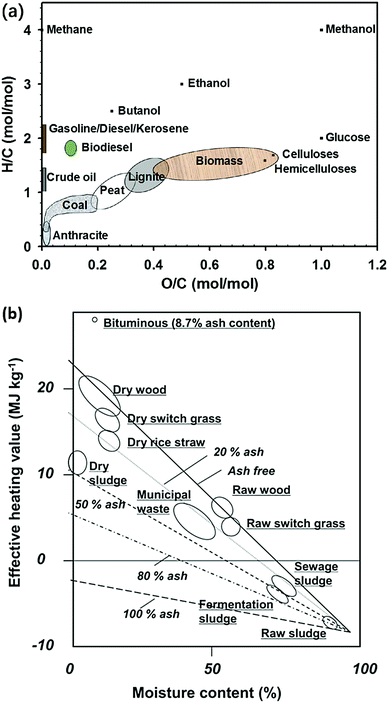 | ||
| Fig. 2 (a) van Krevelen diagram for various fuels. Adapted from ref. 38 and 39. (b) Effect of moisture and ash content on the effective heating values of various biomass. Adapted from ref. 12. | ||
Compared to coal, the moisture content of biomass is much higher, which results in a low heating value/energy density for biomass feedstocks. For biomass, lignin usually has lower oxygen content and higher carbon content than cellulose or hemicellulose and it is believed a higher lignin content corresponds to a biomass with a higher heating value.12,40Fig. 2b depicts the effect of moisture and ash content on the effective heating values of various biomass.
The general advantages of using biomass as a fuel include:
(a) Its renewable nature;
(b) Close to carbon neutral with negative emission potential;
(c) Low ignition temperature;
(d) Generally exceptional low content of pollutants such as sulphur and mercury that are often found in fossil energy sources (i.e., coal); and
(e) Low ash content.
The potential disadvantages of using biomass as a fuel include:
(a) High moisture content;
(b) Low energy density;
(c) Complicated composition and inconsistent feedstock availability;
(d) High alkaline and alkaline earth metals contents;
(e) Low ash melting point;
(f) Uncertainty in collection, transportation and pretreatment costs and
(g) Where a biomass has been grown on degraded or contaminated land, it is possible that it has a significant take-up of heavy metals (in contrast to point (d) in advantages).
Some residual or biomass wastes are favourably used, including (1) non-edible agricultural, forest, feed and food residues; (2) contaminated or industrial biomass; (3) short-rotation energy crops and (4) animal and human waste.40 One particular advantage of biomass fuel in comparison to coal for a CLP system is that biomass contains a relatively higher fraction of volatile matter, which will be discussed below.
Vassilev developed a chemical classification of biomass ash, as shown in Fig. 3, examining 86 types of representative biomass and 38 types of solid fuels.22 Three groups of dominant mineral compositions of biomass ash were identified on the basis of their occurrence, content and origin of biomass. As shown in Fig. 3, the upper corner (Si–Al–Fe–Na–Ti) represents mostly glass, silicates and oxyhydroxides, the left corner (Ca–Mg–Mn) includes commonly carbonates, oxyhydroxides and glass, silicates, and the right corner (K–P–S–Cl) is mostly phosphates, sulphates, chlorides glass and some silicates and carbonates.22 Biomass ash can further be divided into four types, S, C, K. and CK. The C, K, and CK types are mainly responsible for the enhanced leaching behaviour, low-temperature transformation, partitioning or emission of volatile components and deposits during combustion. The more acidic S type accounts for enhanced abrasion–erosion during combustion, and the formation of some low-temperature eutectics which decrease combustion efficiency.22
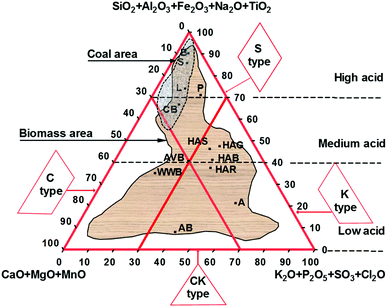 | ||
| Fig. 3 Chemical classification of ashes and composition areas associated with biomass and coal ash. (Average values: A – algae, B – bituminous coal, S – sub-bituminous coal, L – lignite, P – peat, AB – animal biomass, CB – contaminated biomass, AVB – all varieties of biomass, HAB – herbaceous and agricultural biomass, HAG – herbaceous and agricultural grass, HAS – herbaceous and agricultural straw, HAR – herbaceous and agricultural residue and WWB – wood and woody biomass.) Adapted from ref. 22. | ||
The different ash compositions of coal and biomass have significant implications for BCLPs, where coal ash has a softening temperature >1000 °C and a melting point of 1100–1400 °C, which are higher than that most envisaged CLP systems will likely operate.42 Biomass ash can melt at significantly lower temperatures (as low as 800 °C), which causes potential issues due to the agglomeration of oxygen carriers.
2.2. Conventional thermochemical conversion of biomass
The current pathways for energy/fuel conversion from biomass can be classified into biochemical and thermochemical processes.11,25 Typical biochemical approaches include fermentation, anaerobic digestion and biophotolysis.12 Biochemical method, in comparison to thermochemical conversion, are potentially more environmentally friendly and less energy intensive; however, they also have very slow kinetics, low energy/fuel conversion efficiency,43,44 and more stringent biomass source/property constraints. Moreover, biochemical processes using microorganisms consume a large quantity of water. Furthermore, some cellulosic biomasses are not convertible via this route.11,45Generally, the primary thermochemical processes for biomass conversion include combustion, gasification, pyrolysis and chemical looping. These processes are inherently related to each other. An overall summary of these conversions is tabulated in Table 1. It should be noted that carbon capture could be added to the back end of the conventional biomass thermochemical conversion processes to capture CO2 for a net CO2-negative process. For example, post-combustion carbon capture could be combined with biomass combustion. However, the additional carbon capture unit will increase the complexity of the power plant. In addition, even for the current commercial post-combustion carbon capture technology, the electricity cost will increase by up to 80% and the efficiency of the power plant will decrease by 20–30%.46 In comparison, technologies such as biomass-based chemical looping offer higher efficiency and potentially lower cost, and therefore very promising.
| Thermochemical conversion | Combustion | Gasification | Pyrolysis |
|---|---|---|---|
| Purpose | Converting biomass to heat and electricity | Converting biomass to high HV gas | Converting biomass to biochar and bio-oil |
| Atmosphere | Oxidizing atmosphere (oxidant usually higher than the stoichiometric value) | Partial oxidizing atmosphere (oxidant lower than the stoichiometric value) | No oxidant |
| Reaction medium | Air | Air, pure oxygen, steam, and CO2 | None |
| Temperature | 700–1400 °C | 500–1300 °C | 380–830 °C |
| Gas products | CO2 and H2O | CO, H2, CO2, H2O, and CH4 | CO, H2, CH4 and other hydrocarbons |
| Pollutants | SOx, NOx, polycyclic aromatic hydrocarbons (PAHs), and dust | H2S, NH3, tar, and dust | H2S, NH3, tar, and dust |
| Advantages | Process is relatively simple. Co-combustion of biomass and coal do not need changes to current power plants. | Production of a variety of chemical products, such as methanol and other hydrocarbons, allows for flexible adaptation to market conditions. | Liquid fuels are directly produced, which after appropriate treatment may be directly treated in conventional refineries. |
| Disadvantages | NOx, SOx, and particulates are formed during combustion.48 Other potential unburnt pollutants, such as CO, PAHs, condensed fumes (tars/oils), soot, and unburnt carbon also have negative environmental implications.49 Elements including Si, K, Na, S, Cl, P, Ca, Mg, and Fe present in biomass can cause ash fouling and slagging (ash fusion).50 | Tar can block downstream processes and lower gasification efficiency.51 | High energy consumption is inevitable for pyrolysis due to its endothermic nature, and further research is needed before its industrial scale implementation.12 |
2.3. Biomass as a fuel for chemical looping processes
Fuels in various forms can be used as feedstocks for CLPs. Gaseous and solid fuels are the most commonly used forms, whereas very limited studies have been reported on the use of liquid fuels.47 With gaseous fuels, solid LMs can react directly with the fuel through intensive gas–solid interactions resulting in high conversion efficiency, whereas with the use solid fuels, the direct solid–solid interactions between LMs and solid fuels are extremely slow and solid LMs can only react with the released volatiles or gasified components.Methane/natural gas is the most studied fuel for CLPs and its predominant reactions are heterogeneous gas–solid reactions between fuel and oxygen carriers (OCs). When fuelled by coal, the chemical reactions involved are (gaseous phase mediated) heterogeneous solid–solid reactions, which can be extremely slow. The reported coal conversion and CO2 concentration in the fuel reactor are significantly lower than that in gas-fuelled chemical looping combustion (CLC) systems.11,52,53 Due to the relatively low reactivity of coal, the development of solid fuel use for chemical looping processes has been stagnant for some time.54
As a renewable energy resource, biomass can act as a potential alternative to fossil fuels. Moreover, the key advantages of biomass in comparison to coal in CLC systems include its greater overall reactivity, its char allows more rapid complete burnout, and lower potential for transfer of carbonaceous material to the metal/air reactor, where it would burn and release CO2.42 Some other aspects associated with CLPs for various forms of fuels are summarized in Table 2.
| Gaseous fuel | Solid fuels | ||
|---|---|---|---|
| Natural gas | Coal | Biomass | |
| Net CO2 effect w/o CCS | Carbon positive | Carbon positive | Nearly carbon neutral |
| Net CO2 effect w/ CCS | Carbon neutral | Carbon neutral | Carbon negative (BECCS) |
| Fuel reactivity | High | Low | High |
| Gasification temperature | Not applicable | High | Low |
| Interactions between fuel and looping materials | Direct solid–gas interactions |
Direct solid–solid interactions (extremely slow);
Volatiles or gasified components for solid–gas interactions. |
Direct solid–solid interactions (extremely slow);
Volatiles or gasified components for solid–gas interactions. |
| Fuel-LMs contact efficiency | High | Low | Low |
| Solid circulation rate | Low | High | High |
| Influence of fuel-derived sulphur on LMs | Low | High | Low |
| Influence of fuel-derived alkaline/earth alkaline metals on LMs | No | Low | High |
| Influence of tar on the system | No | Low | High |
| Influence of ash melting on the system | No | Low | High |
| Separation of ash and looping materials | Unnecessary | Necessary | Necessary |
| Pre-drying of fuel | Unnecessary | Unnecessary | Depends on biomass moisture content and CLPs types |
3. Innovative schemes of biomass-based chemical looping technologies
Compared to other CCS technologies, CLPs can achieve higher energy efficiency due to their inherent avoidance of gas separation steps. Early research on CLPs mainly focused on gaseous fuels, while CLPs based on solid fuels experienced important developments in the last decade.29 In addition, biomass-based CLPs, i.e. BCLPs, are attracting growing interest as an effective approach to realise BECCS. Moreover, if biomass waste (such as sludge from wastewater treatment plants) can be utilised to produce energy, BCLPs can provide a route for waste to energy.In any system, the maximum amount of usable work during a transformation to equilibrium with regards to a chosen reference state is called exergy.14,55 CLP is an emerging technology, which has the dual advantages of minimising exergy loss and simplifying product separation. As shown in Fig. 4a, in a typical CLP, the overall reaction (eqn (1)) can be divided into two sub-reactions (eqn (2) and (3)) occurring in two separate reactors. The looping material in the form of LM1 is transformed into LM2 after reacting with A (eqn (2)) followed by the regeneration of LM2 in the other reactor (eqn (3)) making a closed loop with the interlinked reactors. Moreover, the products of eqn (1), C and D, are separated in two sub-reactions. It is noteworthy that when using a solid LM, the CLP is predominantly a series of gas/solid reactions or even (gas mediated) solid/solid reactions.
| A + B → C + D | (1) |
| A + LM1 → C + LM2 | (2) |
| B + LM2 → D + LM1 | (3) |
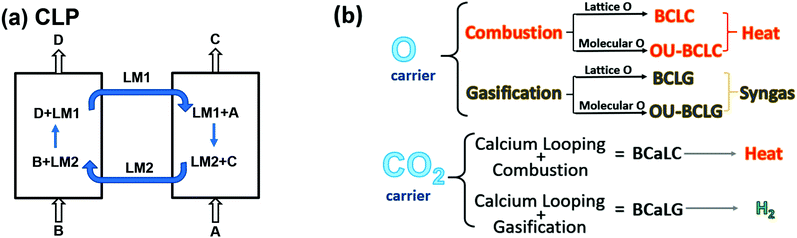 | ||
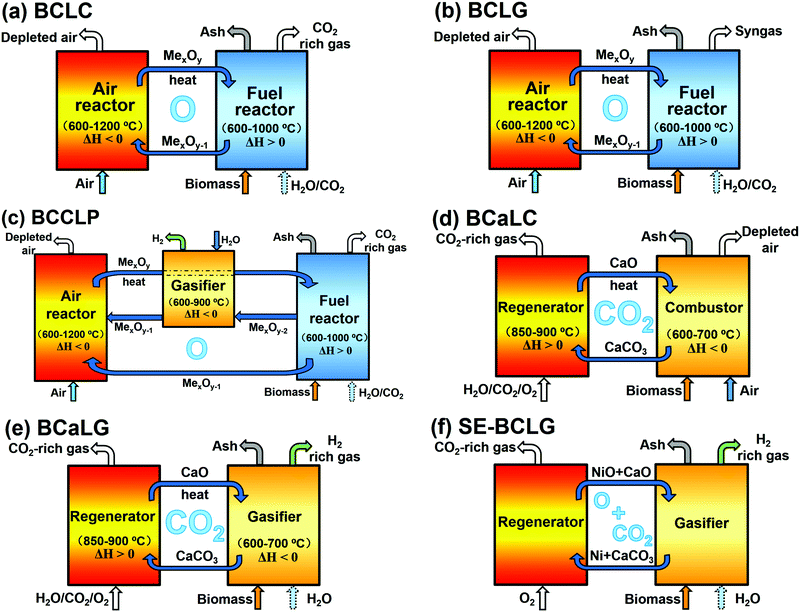
| Fig. 4 (a) Schematic principles of chemical looping, and (b) classification basis for typical biomass-based CLPs. | ||
Similar to CLPs, BCLPs can also be classified on the basis of LMs, i.e. oxygen carrier, OC, and CO2 carrier, CC (Fig. 4b).14 OC can transfer O by providing lattice oxygen in the processes of biomass-based chemical looping combustion (BCLC) and biomass-based chemical looping gasification (BCLG) or releasing molecular oxygen in the processes of oxygen uncoupling-BCLC (OU-BCLC) and oxygen uncoupling-BCLG (OU-BCLG). When CO2 carriers serve as LMs, biomass gasification is enhanced by a recyclable CO2 acceptor, which is usually CaO. Taking advantage of the typical calcium looping (CaL), in situ carbon capture can be realised in the gasifier and a CO2-rich stream can be produced in the calciner/regenerator. Similarly, the process mainly producing heat can be termed biomass calcium looping combustion (BCaLC), whereas the process mainly producing gas is termed biomass calcium looping gasification (BCaLG).
The BCLPs can also be categorized based on the target output as follows:
(a) To generate heat/electricity. This type of process refers to combustion, as shown in Fig. 5a (BCLC) and Fig. 5d (BCaLC);
(b) To generate fuels such as H2 or syngas. This type is usually referred to as a gasification or reforming process, as illustrated in Fig. 5b (BCLG) and Fig. 5e (BCaLG).
(c) To co-generate heat and gas. When the fully reduced OCs from the fuel reactor are oxidised successively by H2O and air, H2 and heat could be generated, respectively. Such technology combines the production of electricity and fuels, and is thus referred to as biomass-based co-production chemical looping process (BCCLP), as shown in Fig. 5c.
Moreover, OCs and CCs can co-exist in one system with dual loops, as shown in Fig. 5f, which is usually termed as sorption-enhanced BCLG (SE-BCLG).
3.1. Biomass-based chemical looping combustion (BCLC)
BCLC processes can convert biomass and produce a pure stream of CO2. In a typical BCLC process, a metal oxide (MexOy) and its reduced form (MexOy−1) serve as an OC, which transports oxygen between the air reactor (or oxidiser) and fuel reactor (or reducer), as shown in Fig. 5a. In the fuel reactor, MexOy reacts with biomass (CnH2mOp) to produce CO2, H2O and MexOy−1 (eqn (4)), whereas in the air reactor, MexOy−1 is oxidised, as illustrated in eqn (5).| CnH2mOp + (2n + m − p)MexOy → nCO2 + mH2O + (2n + m − p)MexOy−1 | (4) |
| (2n + m − p)MexOy−1 + (n + 0.5m − 0.5p)O2 → (2n + m − p)MexOy | (5) |
Usually (with some exceptions), the reactions based on eqn (4) are endothermic and the reactions based on eqn (5) are exothermic. The net energy balance of the whole system for an ordinary combustion system, combining eqn (1) and (2), can yield a complete ordinary combustion (eqn (6)):
| CnH2mOp + (n + 0.5m − 0.5p)O2 → nCO2 + mH2O | (6) |
In the BCLC route, the OC is the key material that circulates within the two reactors thus avoiding direct contact between the fuel and air. Almost pure CO2, which can be readily captured, is obtained from the fuel reactor. Moreover, compared to the traditional combustion processes, BCLC can greatly reduce NOx emissions and enhance thermal efficiency.54
There are two approaches to realise BCLC: (1) gasify biomass to form syngas and then use it for CLC. However, an additional gasifier is required (both increasing Capex significantly and leading to unfavourable economics56,57) to produce undiluted syngas. (2) Directly introduce biomass into the fuel reactor. Sometimes, biomass is gasified in situ by H2O or CO2 as the gasification agent, which is termed in situ gasification BCLC (iG-BCLC), as shown in Fig. 6a. Two reaction paths are proposed between the OC and biomass in the fuel reactor: (a) direct reduction of OC by biomass, and (b) reduction of OC by the gaseous biomass gasification product. The first path has two components, reactions of the volatile matter ejected from the fuel with the OC and direct solid–solid reactions. The relatively high volatile matter composition of biomass and high reactivity of biomass tar in comparison to high-rank coals yield an advantage for biomass in this context in that a greater proportion of the fuel can directly reduce the oxygen carrier in a CLC system, as opposed to reacting indirectly through an intermediate gas-phase species such as CO or H2. Solid–solid reactions are generally limited, owing to the low solid/solid contact efficiency and are usually considered unlikely to occur at an appreciable rate.57 In the second path, biomass is gasified with H2O/CO2 to yield mainly H2/CO, and the produced syngas can readily react with the OC. In this study, BCLC refers to iG-BCLC if there is no specific reference.
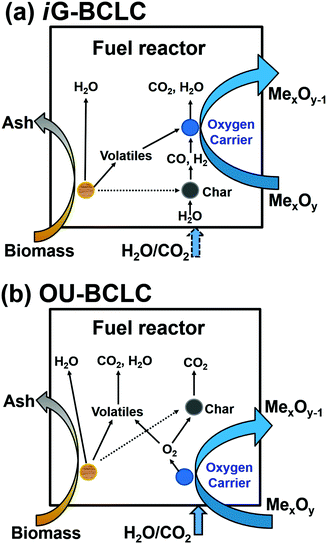 | ||
| Fig. 6 Main reactions in the fuel reactor for solid fuels: (a) iG-BCLC (referred to as BCLC in this article) and (b) OU-BCLC. | ||
The direct use of biomass as a fuel has been extensively investigated. Key information comparing the representative cases of BCLCs is summarised in Table 3. In a 1.5 kWth process (case ICB-2013), pine sawdust was used as a fuel and iron ore was used as the OC.58 Small amounts of CO, H2 and CH4 were detected as unburned compounds. Tar (mostly naphthalene) production was reported to decrease at high fuel reactor temperatures. Carbon capture efficiencies (>97.5%) were obtained in the temperature range of 880–915 °C using either steam or CO2 as the gasifying agent.58 Also in the case of SEU-2009, synthesised iron oxides were used with pine sawdust in a 10 kWth process.53 Higher fuel reactor temperatures led to a greater increase in CO production than the consumption of CO in the oxidation to CO2 alone. The reduction of Fe2O3 to Fe3O4 was utilised for iron oxide reduction with biomass syngas.53 More recently, in cases SEU-2015a and 2015b, dewatered sewage sludge was investigated for CLC59,60 using Fe-based or Ni-based OCs. Increasing the fuel reactor temperature intensified the gasification step and the subsequent reduction process, thus increasing the carbon conversion and combustion efficiency. Over 10 h of continuous operation the reactivity of hematite only slightly decreased, which illustrates that CLC could be an alternative treatment technology for sewage sludge. BCLC can also be integrated with torrefaction processes using produced volatiles as feedstock with high energy conversion efficiency for the overall processes reported.61,62 In this way, biomass is indirectly utilised and thus will not be discussed in detail in this review.
| Case no. (size) | Biomass | Oxygen carrier | Fuel reactor | Air reactor | Efficiency and remarks |
|---|---|---|---|---|---|
| a Case no: CUT: Chalmers University of Technology, Sweden; SEU: Southeast University, China and ICB: Instituto de Carboquímica (ICB), Spain. | |||||
| CUT-2013 (12 MWth)68,69 | Wood chips (Ash: 26% Ca, 12% K, 4% Mg, 2% Si, etc.) | Nature ore ilmenite (mainly FeTiO3) | Circulating fluidised boiler with oxidation/redox cycle within the main boiler. |
• Operational time: 96 h.
• As compared with the blank case, up to 30% NO reduction was observed when 40% ilmenite was introduced as an oxygen carrier. • Potassium was found to be the key problematic ash compound. Homogeneously diffused potassium was observed with KTi8O16 formed. |
|
| ICB-2013 (1.5 kWth)58 | Pine sawdust (Ash: 41% CaO, 9% K2O, 7% MgO, 7% SiO2) | Iron ore (76% as Fe2O3) | Bubbling fluidised bed, fluidised by steam or CO2. Operation temperature: 880–915 °C | Fluidised bed, fluidised by nitrogen. |
• Operational time: 78 h.
• A small quantity of CO, H2 and CH4 was detected as unburned compounds. Tar content decreased at high fuel reactor temperatures. Carbon capture efficiencies of more than 97.5% were obtained in 880–915 °C both using steam and CO2 as gasifying agents. |
| SEU-2009 (10 kWth)53 | Pine sawdust | Iron oxide (mainly Fe2O3) | Spout-fluidised bed. Operated at 740–920 °C. Fluidising agent: CO2. Operation temperature: 740–920 °C | Fast fluidised bed |
• Operational time: 30 h.
• Higher temperatures in the fuel reactor helped to increase CO production from biomass gasification. The transformation of Fe2O3 to Fe3O4 is the favoured step in the process of iron oxide reduction with biomass syngas. |
| SEU-2011 (1 kWth)70 | Sawdust | Natural iron ore (81% as Fe2O3 and 15% as SiO2) | Spout-fluid bed-rectangular bed. Fluidised with steam and N2. Operation temperature: 740–925 °C | High-velocity fluidised bed. | • CO2 capture efficiency: 95.25% at 720 °C and ∼98.6% at 925 °C. At a higher fuel reactor temperature, a lower CO2 concentration was produced. The poor oxygen transport capacity and the thermodynamic constraint of the iron ore limited the conversion efficiency of carbonaceous gases. |
| SEU-2015a60 | De-watered sewage sludge (Ash: 31% Si, 16% Al, 14% P, 17% Fe, 11% Ca, etc.) | Ni-based material (20% NiO, 39% NiAl2O4 and 41% Al2O3) | Batch fluidised bed reactor. Fluidised with steam and N2. Operation temperature: 700–900 °C. | Batch fluidised bed reactor. | • The nickel-based oxygen carrier enhanced the overall carbon conversion and fuel conversion rate. When compared to bituminous coal as the feedstock, a higher carbon conversion and fuel conversion rate were obtained for sewage sludge (at 700–900 °C). Lower CO2 capture efficiency was reported (75–84%) for sewage sludge than coal (around 82–92%). No sintering/agglomeration issues were reported during 20 redox cycles. |
| SEU-2015b (1 kWth)59 | De-watered sewage sludge (Ash: 36% Si, 20% Fe, 15% Al, 11% P, etc.) | Hematite (83% as Fe2O3) | Spout-fluid bed reactor. Fluidised with steam. Operation temperature: 800–925 °C. | Fast fluidised bed |
• Operational time: 10 h.
• Increasing the fuel reactor temperature intensified the gasification step and the subsequent reduction process leading to an increase of carbon conversion and combustion efficiency. During 10 h continuous operation, hematite showed a slight decrease in reactivity. |
| ICB-2014 (1.5 kWth)66,67 | Pine wood chips. (Ash: 41% CaO, 9% K2O, 7% MgO, 7% SiO2) | Cu-based material, prepared via spray-drying using CuO and MgAl2O4. | Bubbling fluidised bed. Fluidising agent: N2 and CO2. Operation temperature: 860–935 °C. | Bubbling fluidised bed |
• Operational time: 10 h.
• A fuel reactor temperature higher than 900 °C was required to exploit the oxygen uncoupling benefits, resulting in no unburnt compounds at the fuel reactor outlet. The char conversion rate of biomass in the CLOU process was between 3 and 4 times higher than that corresponding to the iG-CLC process at temperatures above 900 °C. |
Carbon capture efficiency (ηcc) represents the removal efficiency of carbonaceous gas that would otherwise be emitted to the atmosphere. This parameter is calculated as the ratio of carbonaceous gas flow leaving the fuel reactor to overall carbonaceous gas outlet stream of CLCs:58
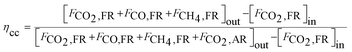 | (7) |
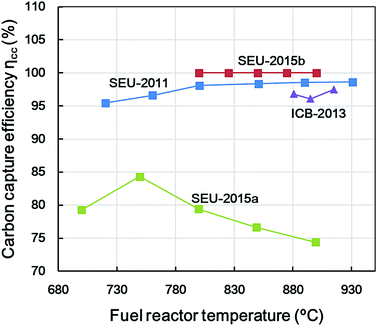
Research groups from Southeast University (SEU, China) and Instituto de Carboquímica (ICB, Spain) adopted this parameter to compare the carbon negativity of the BCLC processes. The reported ηcc is summarised in Fig. 7 as a function of the working temperature of the fuel reactor.
 | ||
| Fig. 7 Carbon capture efficiency comparison as a function of fuel reactor temperature for the different cases. | ||
As shown in Fig. 7, all the ηcc are higher than 95% for cases SEU-2011, 2015b and ICB-2013. The ηcc increased at a higher temperature for all three cases. It is noteworthy that dewatered sewage sludge was used as biomass feedstock in SEU-2015b and the ηcc reached almost 100% at 800–900 °C, which indicates that no char bypassed to the air reactor.59 For case SEU-2015a, which was also sewage-sludge-based, the efficiency oscillated between 75% and 84%. The different reactor designs, OCs used, and ash properties may contribute to this discrepancy.
Primary, secondary and tertiary tars can be produced during biomass pyrolysis and this tar classification is also applicable for BCLP studies.63 Primary tars are mainly cellulose, hemicellulose or lignin-derived products. Secondary tars are characterised by olefins and phenolics whereas tertiary tars are comprised of methyl derivatives of aromatics (methylnaphthalene, toluene, indene and phenol) and polycyclic aromatic hydrocarbons (PAHs) without substituents (benzene, naphthalene, anthracene, phenanthrene and pyrene). With elevated temperatures, the produced tars can be converted into light hydrocarbons thereby shifting from primary or secondary tars to tertiary tars. Heavier tars are more difficult to gasify, even at high temperatures. Less styrene, indene and naphthalene were found to be produced at higher operation temperatures, which indicate higher reforming and catalytic reactivity at higher temperatures.58
| Biomass → volatile matter + char/ash | (8) |
| 2MexOy → 2MexOy−1 + O2 | (9) |
| Volatile + O2 → CO2 + H2O | (10) |
| Char + O2 → CO2 + ash | (11) |
Similarly, in iG-BCLC, after steam condensation, pure CO2 can be obtained from the exit gas of the fuel reactor. The processes of OU-BCLC and iG-BCLC share some similar difficulties, including separation of OCs from residue solids, carbon deposition and OCs deactivation. Moreover, only a limited number of metal oxides can meet the requirement for multiple cycles of oxygen uncoupling processes.27
OU-BCLC is a relatively new concept and to the best of our knowledge, only a few related studies are available, in which OU-CLC was mostly performed with coal. In case ICB-2014 (last entry of Table 3), pine wood chips with a heating value of 19.2 Mg kg−1 were used in a CuO OU-BCLC,66 and higher temperatures improved the oxygen uncoupling effect (as expected by thermodynamics). A fuel reactor temperature >900 °C resulted in O2 production and no unburnt compounds at the fuel reactor outlet. The biomass char conversion rates were around 3 to 4 times higher than the corresponding iG-BCLC processes at >900 °C.66 In the comparison of iG-BCLC and OU-BCLC in a continuous 1.5 kWth BCLC unit, the OU-BCLC technology presented the advantage of less tar at the outlet of fuel reactor.67 Meanwhile, OU-BCLC generated a lower quantity of unburned products, such as H2, CO, and CH4, which decreased with an increase in temperature, as shown in Fig. 8.
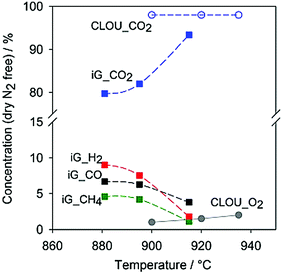 | ||
| Fig. 8 Gas products from fuel reactors of iG-BCLC (iG in the figure) and OU-CLC (CLOU in the figure) at different temperatures. Reproduced from ref. 67 by permission from John Wiley & Sons Ltd. | ||
3.2. Biomass-based chemical looping gasification (BCLG)
BCLG shares similar principles with BCLC, as illustrated in Fig. 5b.6 However, in contrast to BCLC, BCLG can produce useful combustible gas. Biomass is partially oxidised (to produce a mixture of H2 and CO, syngas) as opposed to being fully oxidised in BCLC (where the desired product is heat and electricity from the heat). The reactions in the air reactor for BCLG and BCLC (eqn (5)) are the same, but in the fuel reactor, oxidation of the fuel (eqn (4)) occurs to a smaller extent6,16 with the predominant reaction being partial oxidation (eqn (12)) which produces syngas.| CnH2mOp + (n − p)MexOy → nCO + mH2 + (n − p)MexOy−1 | (12) |
It should be noted that steam or CO2 might be added into the fuel reactor to enhance steam reforming (eqn (13)) and CO2 reforming (eqn (14)). In this case, BCLG can also be called chemical looping reforming (BCLR).
| CnH2mOp + (n − p)H2O → nCO + (m + n − p)H2 | (13) |
| CnH2m + nCO2 → 2nCO + mH2 | (14) |
Reactions (12) and (13) are strongly endothermic, and thus require an external heat supply to the fuel reactor. The major advantages of CLG include avoidance of direct air-fuel mixing, the availability of heat for CH4-to-H2 conversion without costly oxygen production, and higher H2 production efficiency. The inorganic species present in biomass ash are effective gasification catalysts,42 which is a potential advantage for CLG with biomass, as opposed to coal.
The feasibility of various types of biomass-based BCLG using different OCs has been intensively investigated. Typically, woody biomass is used, and the commonly used OCs include Fe-based,71–73 Fe-ore,74–77 Ni-based,78–80 Ni modified Fe-ore,76 Cu-based,81 Cu-ore,82 and Fe–Ni bimetallic OCs.82 Similar to the concept of OU-BCLC, appropriate OCs can release gaseous O2 to partially oxidize the biomass feedstock and this process can be termed OU-BCLG. Partial oxidation can also be achieved by using OCs suitable for OU-BCLG, yet only one case was reported in the literature.83 Representative cases of BCLG and OU-BCLG are compared and tabulated in Table 4.
| Case no. (size) | Biomass | Oxygen carriers | Fuel reactor | Air reactor | Efficiency and remarks |
|---|---|---|---|---|---|
| a Case no: CQU: Chongqing University, China; HUST: Huazhong University of Science and Technology, China; GIE: Guangzhou Institute of Energy Conversion, China and SEU: Southeast University, China. | |||||
| HUST-201581,84 | Pine sawdust |
Copper ore (mainly CuO, CuFe2O4)
Hematite (mainly Fe2O3, Al2O3, SiO2) synthesised CuO/CuAl2O4 or Fe2O3/Al2O3 |
Fluidised bed reactor, with N2 atmosphere as fuel reactor conditions and air atmosphere as air reactor conditions.
Operation temperature: 800 °C. |
• Gas yield (N m3 kg−1): CuO/CuAl2O4 (0.90) > Fe2O3/Al2O3 (0.82) > copper ore (0.79) > hematite (0.78).
• Carbon conversion efficiency: CuO/CuAl2O4 (95.6%) > copper ore (83.2%) > Fe2O3/Al2O3 (81.7%) > hematite (64.6%). • Gasification efficiency: Fe2O3/Al2O3 (60.1%) > hematite (55.1%) > CuO/CuAl2O4 (30.8%) > copper ore (26.6%). • The Cu-based materials have higher reactivity for biomass pyrolysis and gasification, resulting in relatively higher carbon conversion and more tar-cracking. |
|
| GIE-201372,74,75 | Pine sawdust | Natural hematite (91% as Fe2O3) | Bubbling fluidised bed. Fluidising agent: Ar/steam. Operation temperature: 740–940 °C. | Bubbling fluidised bed | • The maximum gas yield of 1.06 N m3 kg−1 and highest gasification efficiency of 83.31% were reached when a Fe2O3/C molar ratio of 0.23 was used. The oxygen carrier was gradually deactivated with an increase in reduction time owing to the loss of reactive lattice oxygen. Agglomeration and attrition of oxygen carriers over cycles were observed. |
| GIE-201476,85 | Biomass char | Iron ore (90% as Fe2O3)76 or NiO-modified iron ore85 | TGA reactor, with argon/steam/CO2 atmosphere as fuel reactor conditions and air atmosphere as air reactor conditions. Operation temperature: 600–1200 °C. |
• The overall reactivity of oxygen carriers increased with the loading of NiO. The presence of spinel-type NiFe2O4 greatly enhanced the char gasification. The carbon conversion reached up to 55.56% compared with char pyrolysis (5.52%).76
• The oxidising atmosphere (CO2, or H2O) resulted in an increase in carbon conversion efficiency and suppressed oxygen conversion of the oxygen carrier. The order of reactivity is speculated as follows: pure oxygen > NiO > H2O > iron ore > CO2 > Al2O3.85 |
|
| GIE-2015 (10 kWth)71,82 | Pine sawdust |
Fe2O3/Al2O3 (mass ration = 7/3)71
Fe2O3/Al2O3/NiO (mass ration = 7/3/0.53)82 |
Bubbling fluidised bed. Fluidising agent: N2. Operation temperature: 650–900 °C. | Fast fluidised bed. |
• Operational time: 60 h.
• Higher operating temperatures in fuel reactor resulted in higher syngas yield, cold gas efficiency, and carbon conversion. The synthesised oxygen carriers exhibited stable reactivity and resistance to agglomeration over 60 h operation.71 • The NiO-modified oxygen carriers showed higher gasification efficiency due to the synergistic effect between Fe2O3 and NiO. In addition, the modified oxygen carriers performed well over 11 cycles with good crystalline state.82 |
| GIE-201686 | Biomass char | Iron ore (90% as Fe2O3) | Fixed bed reactor, with steam/N2 atmosphere as fuel reactor conditions and air atmosphere as air reactor conditions. Operation temperature: 850 °C. |
• Operational time: 52 h (20 cycles).
• The overall char conversion rate increased with steam content in fuel reactor and reached a maximum at a steam content of 56.6%. The oxygen carriers maintained relatively stable reactivity after 20 cycles and a slight decrease in carbon conversion was reported. |
|
| SEU-2015 (25 kWth)80 | Rice straw | NiO/Al2O3 (mass ratio = 3/2) or with CaO addition (10%) | Bubbling fluidised bed. Fluidising agent: steam/N2. Operation temperature: 650–850 °C. | High-velocity fluidised bed. | • The carbon conversion efficiency increased from 40.55% to 67.5% when the temperature of the fuel reactor increased from 650 °C to 850 °C. The syngas yield reached a maximum of 0.33 N m3 kg−1 at 750 °C. CaO decoration can enhance the quality of syngas with low CO2 emission. |
| SEU-2016a (25 kWth)77 | Rice husk | Natural hematite (83% as Fe2O3) | Bubbling fluidised bed. Fluidising agent: steam/N2. Operation temperature: 800–900 °C. | High-velocity fluidised bed. | • The carbon conversion efficiency increased from 53.4% to 89.2% when the working temperature of the fuel reactor increased from 800 °C to 900 °C. The syngas yield reached the maximum of 0.74 N m3 kg−1 at 860 °C. |
| SEU-2016b87 | Rice husk | Natural hematite (83% as Fe2O3) | Batch fluidised bed reactor, with steam/N2 atmosphere as fuel reactor conditions and air atmosphere as air reactor conditions. Operation temperature: 750–900 °C. | • In the batch reactor, carbon conversion efficiency increased within the tested temperature range. The hematite fraction posed a similar effect on the gasification performance between the batch reactor and the continuous reactor. | |
| CQU-201683 | Walnut shell | CuO supported on MgAl2O4 | Tubular fixed bed reactor with switchable gas atmosphere: with N2 atmosphere as fuel reactor conditions and air atmosphere as air reactor conditions. Operation temperature: 600–1000 °C. | • Compared to pure CuO, the addition of MgAl2O4 can effectively improve the stability and reactivity resulting in a higher yield of syngas. | |
In a pine sawdust-fuelled CLG (case HUST-2015), compared to Fe-based OC, Cu-based particles provided higher gas yield and carbon conversion efficiency but a lower cumulative concentration of gaseous C2Hm and tar. The amount of tar produced decreased at higher temperatures on account of enhanced tar cracking.81 In another study (case GIE-2013), Fe2O3/Al2O3 was used as OCs with pine sawdust.72 Higher temperatures produced more CO and H2, less residual char in the fuel reactor and reduced CO2 concentration in the exhaust from the air reactor. The carbon conversion rate and gasification efficiency increased with an increase in temperature, and H2 production was maximum at 870 °C.72 In a biochar-fuelled BCLG (case GIE-2014), a higher carbon conversion (55.56%) was obtained in comparison to the baseline experiment without OCs (5.52%).76 Biomass char was catalytically pyrolysed because of the presence of deeply reduced products (metallic iron and nickel) which act as catalysts for char pyrolysis.76
Temperature usually plays an important role in the BCLG processes. The influence of temperature on the gas properties and efficiencies in a 10 kWth interconnected fluidised bed reactor (case GIE-2015) is shown in Fig. 9. The total gas yield, and gas low heating value (LHV) increased with an increase in temperature from 670 °C to 900 °C. In addition, the carbon conversion and cold gas efficiency also increased with the increase in temperature.71 The reason for this is that high temperatures promote the cracking of tars from biomass gasification.51
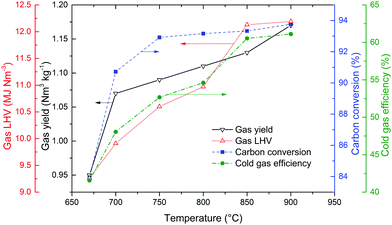 | ||
| Fig. 9 Influence of temperature on the working properties of BCLG.71 | ||
Steam is usually supplied to the fuel reactor as a gasifying agent to accelerate biomass gasification. Steam also provides oxygen for biomass gasification. In case SEU-2016a, carbon the conversion efficiency increased initially and then remained nearly unchanged.77 The optimal S/B ratio was determined as 1.0 for the highest syngas yield without sacrificing the maximal carbon conversion efficiency.
3.3. Biomass-based co-production looping process (BCCLP)
Some OCs, e.g. Fe-based materials, have several oxidation states and can be transformed sequentially into different forms in different reactors. In the BCCLP process, a gasifier is added between the typical air reactor and fuel reactor, and H2 is produced from this gasifier rather than from the biomass conversion reactor (fuel reactor),11,88,89 as shown in Fig. 5c. It should be noted that co-production CLP is a pathway to produce H2, which serves a different market to syngas. In the fuel reactor, MexOy is reduced to MexOy−1 by CO and H2 from biomass gasification, and CO and H2 are then converted to CO2 and H2O. This reaction is the same as reaction (4) of BCLC.In the steam reactor (gasifier), MexOy−1 is oxidised by H2O to MexOy, and pure H2 is generated at the same time. The reaction is
| MexOy−2 + H2O → MexOy−1 + H2 | (15) |
There are only a few studies of BCCLP and in these cases FeO/Fe3O4/Fe2O3 materials are the LMs. The cycle could be FeO–Fe3O4–FeO or FeO–Fe3O4–Fe2O3–FeO depending on whether extra Fe3O4 oxidation by air is introduced.90
The reactions in the fuel reactor (including biomass gasification and Fe2O3/Fe3O4 reduction) are endothermic, and thus additional heat is needed. It should be noted that a small amount of Fe may also be generated during this period.91
In the steam reactor, FeO is oxidised to Fe3O4 by steam and H2 is generated. This reaction is exothermic and low temperature is preferred. For Fe2O3/FeO-looping, Fe3O4 is then oxidised to Fe2O3 by air in the air reactor.
| 4Fe3O4 + O2 + 3.762N2 → 6Fe2O3 + 3.762N2 | (16) |
This exothermic reaction can heat OCs up to a very high temperature (1100 °C),11 which is beneficial for the reaction in the fuel reactor. It should be noted that quite pure N2 is the by-product of this process.
Systems utilising Fe3O4/FeO-looping presented higher gasification efficiency (60%) than that using Fe2O3/FeO-based looping (54%). The CO2 sequestration rates of these two pathways were both higher than 90%.90 Modelling of Fe2O3/FeO-looping BCCLP indicated that a high moisture content in biomass leads to a low OC conversion rate, low H2 production, and low energy efficiency. Therefore, biomass with less than 5 wt% moisture is required. From the model, the fuel reactor was required to be operated at approximately 900 °C and the steam reactor at higher than 600 °C, with the combustor 100–450 °C higher than the steam reactor. Overall, the Fe2O3/FeO looping BCCLP had 10–25% higher efficiency than conventional biomass combustion and gasification processes.11
3.4. Biomass-based calcium looping combustion (BCaLC)
During conventional biomass combustion in air, the produced CO2 is mixed with N2. The excess air coefficient is usually higher than 1 for the complete combustion, so there will be some excess O2 in the fuel gas, as shown in eqn (17).| Biomass + O2 + N2 → CO2 + H2O + O2 + N2 | (17) |
CO2 could be captured in situ, where CaO is frequently used for the capture through the carbonation reaction.92–95
| Carbonation: CaO + CO2 → CaCO3 | (18) |
It should be noted that, this reaction is exothermic and is favoured at low temperatures. Therefore, the combustion must take place at a relatively low temperature (∼700 °C).92,93
In the calciner/regenerator, CaCO3 is converted to CaO at a temperature higher than 800 °C.
| Calcination: CaCO3 → CaO + CO2 | (19) |
CaO is then transferred back to the fuel reactor to close the cycle, as shown in Fig. 5d.92–95
In a 30 kW interconnected fluidised bed reactor, biomass combustion with in situ carbon capture by CaO was tested. The CO2 capture efficiencies were higher than 80%. CO and CH4 were detected, perhaps due to pulsed feeding or an inappropriate air/fuel mixture.93
Further, the concept of BCaLC was experimentally tested in a 300 kWth pilot reactor at 700 °C. According to the authors, a combustion efficiency close to 100% and carbon capture efficiency between 70 and 95% could be achieved using wood pellet as the fuel.94
A large-scale dedicated biomass power plant with CaO for in situ CO2 capture was also modelled by the same group. With a heat exchanger network, this system could have a higher net power generation efficiency compared to oxy-fuel biomass combustion. With a CO2 purification and compression process, a CO2 stream with purity >95 mol% could be obtained.95
3.5. Biomass-based calcium looping gasification (BCaLG)
In a typical BCaLG, as shown in Fig. 5e, the CO2 produced during the steam gasification can be captured in situ by CaO inside the gasifier. Although group I (alkali metals) hydroxides are more effective to produce high purity H2 with in situ carbon capture than group II (alkaline earth metals) hydroxides,96 more research utilises CaO as a sorbent because of its lower cost and relatively easier recovery. The advantages of BCaLG are numerous compared to other pathways. The CO concentration in the produced gas is quite low, and thus the gas meets the requirements for fuel cell applications. Additionally, any H2S and HCl from biomass gasification can also be removed in situ by CaO.97| C + H2O → CO + H2, ΔH298 = +131.3 kJ mol−1 | (20) |
| CO + H2O → CO2 + H2, ΔH298 = −41.2 kJ mol−1 | (21) |
| CaO + CO2 → CaCO3, ΔH298 = −178.3 kJ mol−1 | (22) |
Reaction (20) is strongly endothermic, and generally is significant at high temperatures (>1000 °C), whereas reaction (21) is exothermic and usually requires a catalyst at a low temperature (<400 °C), and reaction (22) generally operates between 600–750 °C at atmospheric pressure. Traditional H2 manufacturing splits reactions (20) and (21) into separate reactors, since the temperature difference between the different reactions lowers the system efficiency. In BCaLG, reaction (22) can provide heat for reaction (20). Meanwhile, the in situ capture of CO2 promotes reactions (20) and (21), which in turn increases the production of H2. At the same time, CaO catalyses the gasification process and tar reforming, which improves the reaction rate and gaseous product formation according to reaction (23):98
| Tars + H2O → CO + H2 + CO2 + hydrocarbons + ⋯ | (23) |
This concept allows a single-loop process with in situ CO2 capture for atmospheric biomass steam gasification to yield relatively pure H2 gas which can use calcined limestone (or potentially other options such as demolition waste99) as a CO2 sorbent.100 The system energy efficiency can reach 88% with almost complete CO2 capture and an H2 content in the produced reformed gas of up to 71% with negligible CO2.100
Gu et al. performed a thermodynamic analysis of biomass-to-synthetic natural gas (SNG) with BCaLG as the first step of the system.101 At Ca/biomass = 0.83, i.e. a stoichiometric ratio of 1, the CH4 content in SNG was maximised. At S/B = 0.6, the overall energy and exergetic efficiencies reached the maximum. The optimal performances showed that the process is competitive compared to the traditional SNG production process. However, the major limitation in BCaLG using CaO is the deactivation of sorbents due to sorbent sintering and biomass ash.102 An overall summary of representative cases of BCaLG is tabulated in Table 5.
| Case no. (size) | Biomass | CO2 carriers | Gasifier | Regenerator | Efficiency and remarks |
|---|---|---|---|---|---|
| a Case no: AIT: Asian Institute of Technology, Thailand; DU: Dalhousie University, Canada; DUT: Dalian University of Technology, China; UN: the University of Newcastle, Australia and VUT: Vienna University of Technology, Austria. | |||||
| AIT-2014107 | Pine sawdust | Calcined limestone (95.5% as CaO) | Bubbling fluidised bed, fluidised by steam. Operation temperature: 500–650 °C. | Circulating fluidised bed, fluidised by air. Operation temperature: 900 °C. | • The maximum H2 and H2 yield reached up to 78% and 451 mL g−1 of biomass. Compared to the CaO-based bubbling fluidised bed gasification, BCaLG resulted in 15% higher concentration of H2, less tar, and almost double the yield of H2. |
| DU-2009100 | Sawdust | CaO | Bubbling fluidised bed, fluidised by steam. Operation temperature: 500–600 °C. | Circulating fluidised bed, fluidised by CO2. Operation temperature: 800 °C. | • The H2 purity can reach up to 71% for a Ca/C ratio of 1 and S/B ratio of 1.5. About 40% of the CaO can be regenerated at 800 °C for 1 h. |
| DUT-2008102 | Pine sawdust | Mixture of calcined olivine (mainly MgO and SiO) and limestone (mainly CaO) | Fixed bed reactor with steam atmosphere as gasifier conditions and air atmosphere as regenerator conditions. Operation temperature: 650–800 °C. | • The H2 content reached up to 60–70% at a steam/biomass weight ratio (S/B ratio) of 0.38–0.59 and CaO/biomass weight ratio of 20 at the reactor temperature of 700–800 °C. The limestone was deactivated irreversibly after 8 cycles due to the formation of inorganic adhesions. | |
| UN-201299 | Pine sawdust | Concrete and demolition waste (CDW)/calcined limestone (CL)/hydrated Portland cement (HPC) | Pressurized TGA with steam/N2 atmosphere as gasifier conditions and N2 atmosphere as regenerator conditions. Operation temperature: 650–900 °C. |
• Operational time: ∼50 h.
• The CDW behaves similarly to the HPC and CL. The increase in S/C ratio led to an increase in purity of H2. For CDW, the CO2 capture efficiency reached up to 56.4% with high-grade H2 produced. In addition, CDW sorbents were found to be less susceptible to deactivation over the regeneration cycles. |
|
| VUT-2009 (100 kW to 8 MW fuel input)108,109 | Wood | Limestone | Steam fluidised gasifier containing olivine as bed material. Bed temperature: 850–900 °C. | Air fluidised regenerator. | • Significant CO2 removal and an increased conversion H2 was reported. The H2 content reached up to 75% with a low tar content (≤1 g N m−3). |
3.6. Sorption enhanced BCLG (SE-BCLG)
Both OC and CC can be used in one system, as shown in Fig. 5f. Oxidation, steam reforming, water-gas shift reaction and in situ CO2 removal are combined to produce H2 auto-thermally using mixed Ni/CaO.103There have also been some attempts to conduct SE-BCLG using liquid biomass as a feedstock, where both OCs and CCs were used for the looping. In a study on steam reforming of cooking oil, NiO was used as the OC and catalyst, and dolomite was used as a CO2 sorbent. At a relatively low temperature (600 °C), high-purity H2 (>95%) was obtained.104,105 From another study on process simulation, a maximum of 153.4 g H2 kg−1 corn stover was obtained.106
At least three reactors are needed for this system: one for reforming, another for CC regeneration, and the third for OC reduction. Another experimental study reported that NiO cannot be fully reduced to Ni, which indicates that the conditions in the reactors are unable to satisfy the requirements for the ideally full conversion of both NiO–Ni and CaO–CaCO3.103
4. Design of looping materials (LMs)
4.1. Oxygen carrier (OC) providing lattice oxygen: LMs for BCLC, BCLG and BCCLP
From a practical standpoint, ideal OCs in BCLPs should undergo multiple cycles with minimal loss in physical integrity and chemical reactivity. The reduction/oxidation potential of a metal oxide can be predicted using a modified Ellingham diagram, which depicts the standard Gibbs free energies of reactions as a function of temperature. A typical diagram for the comparison of commonly used OCs is shown in Fig. 10a, and the same diagram has been adapted for the selection of OCs in various CLPs.15,16 Three key reactions associated with CO2, H2, CH4 and CO are highlighted as reaction lines 1, 2 and 3.| Reaction line 1: 2CO + O2 → 2CO2 |
| Reaction line 2: 2H2 + O2 → 2H2O |
| Reaction line 3: 2C + O2 → 2CO |
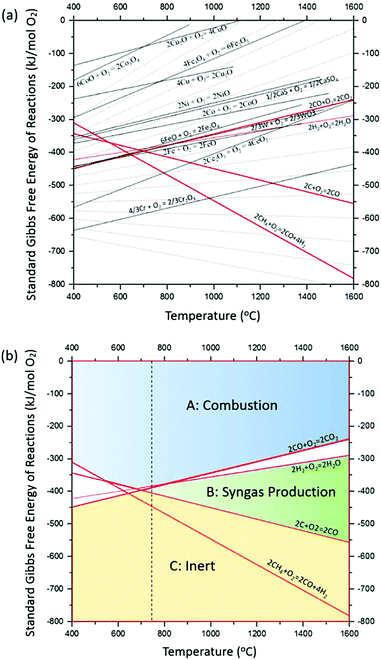 | ||
| Fig. 10 (a) Modified Ellingham diagram for oxygen carrier comparison and (b) zone of metal oxides for chemical looping. Reproduced from ref. 15 by permission from John Wiley & Sons Ltd. | ||
Based on the three key reactions, OCs can fall into three zones/categories according to their potential to fully or partially oxidise the fuel, as shown in Fig. 10b.
Zone A: materials in this zone fall in the area above lines 1 and 2. These materials exhibit strong oxidising potentials and can be used for full/partial oxidation of fuel. Both CO and H2 are readily oxidised. Metal oxides in this zone include NiO, CuO, CoO, Fe2O3 and Fe3O4.
Zone B: materials in this zone fall into the area below lines 1 and 2, but above line 3. These materials can only produce CO or H2, and the yielded syngas cannot be further oxidised. Thus, the materials in this zone are theoretically ideal for partial oxidation of fuel or for CLG.
Zone C: materials in this zone stay in the area below line 3 and are inert for this application.
Materials between lines 1 and 2 are potential choices as partial oxidation materials, which can oxidise H2 into H2O, leaving CO unreacted. For example, SnO2 falls into this area.
For CLC applications, full oxidation is necessary in the fuel reactor, thus potential OCs can be selected from Zone A. The CO2 purity in the exit gas from the fuel reactor reflects the energy conversion efficiencies and commercial viability of the overall CLC systems. According to the reaction thermodynamics, Fe- and Cu-based materials can fully convert fuel into CO2 and H2O, but Ni- or CaS-based materials result in CO leakage.
Partial oxidation can be achieved through two approaches. First, using metal oxides in Zone B to predominantly produce H2 and CO, which cannot be further oxidised due to thermodynamic restrictions. Fig. 10a demonstrates that CeO2 and FeO are representative metal oxides in this zone. The other approach is to utilise sub-stoichiometric quantities of the metal oxides in Zone A. For instance, a CLG process using NiO/Ni, can be operated so that the air reactor is starved, thus producing a mixture of NiO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni (7
Ni (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) instead of fully regenerating all of the Ni to NiO.110 Therefore, less oxygen is transferred to the fuel reactor. Additionally, excess steam can be introduced into the fuel reactor to suppress carbon deposition since Ni is also a strong catalyst for CH4 decomposition.
3) instead of fully regenerating all of the Ni to NiO.110 Therefore, less oxygen is transferred to the fuel reactor. Additionally, excess steam can be introduced into the fuel reactor to suppress carbon deposition since Ni is also a strong catalyst for CH4 decomposition.
Ellingham diagrams can only provide theoretical indications for OCs selection. A combination of reaction kinetics, reactants mixing ratio, contact time and process design determines the actual performance of the system with a given OC.
The typically used OCs are Ni, Fe, Cu, Mn and Co-based materials, among which Fe and Ni-based are the most popular with around 1500 h and 1800 h of operation experience reported, respectively.111 Based on the key information (oxygen transport capacity, melting point, cost, reactivity and resistance to agglomeration or attrition), the OCs are compared in Fig. 11.
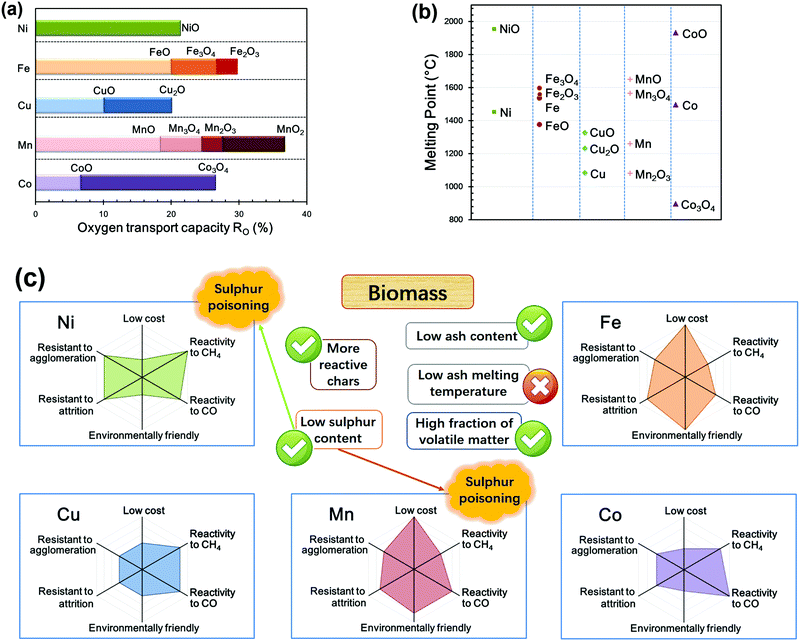 | ||
| Fig. 11 Comparison of Ni, Fe, Cu, Mn and Co-based oxygen carriers: (a) oxygen transport capacity, (b) melting point and (c) summary for cost, reactivity and resistance to agglomeration or attrition. | ||
The oxygen transport capability RO is used to evaluate the maximum oxygen transport between the fully oxidised, mo, and reduced, mr, forms of OC:
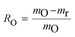 | (24) |
The total cost includes the cost of reactive materials and inert supports (including replacement) and also the manufacturing cost. The general cost of the commonly used metal based materials follows the order of Co > Ni > Cu > Fe > Mn-based materials,6,128 as shown in Fig. 11.
Environmental and health concerns about these materials are also important for the operation and safety of the whole process. In general, Ni- and Co-based materials engender the highest safety concerns during operation. Ni-based materials are potentially carcinogenic in nature. Thus far, the limited focus on the environmental aspects of LMs during their lifecycle has indicated that these are not “immediate showstoppers for the process”.6
In summary, typical OCs based on Ni, Fe, Cu, Mn and Co and their oxides have been developed and intensively investigated and compared. Similar to CLPs fuelled by other feedstocks, Fe- and Ni-based OCs are still the most popular for BCLPs. As shown in Fig. 11c, the low sulphur content of biomass is less of an issue for Ni and Mn-based OCs, which are easily poisoned by H2S or COS. The chars of biomass are more reactive than coal-chars, and the high fraction of volatile matter of biomass makes biomass a more reactive solid feedstock. Although biomass has a low ash content, the low melting point of its ash can cause potential issues with the agglomeration of OCs. More details about the effect of biomass ash can be found in Section 5.1.
4.2. Oxygen carrier (OC) providing molecular oxygen: LMs for CLOU (OU-BCLC and OU-BCLG)
Thermodynamically, a limited number of metal oxides can match the requirements of CLOU processes, especially in consecutive redox cycles. Only the OCs having a suitable equilibrium partial pressure of oxygen gas at the desired temperature range (800–1200 °C) can serve as OCs for CLOU. Thus, far three metal oxide pairs have been proposed: CuO/Cu2O, Mn2O3/Mn3O4, and Co3O4/CoO, and the proposed reversible reactions are:57| 4CuO ↔ 2Cu2O + O2 ΔH850 = 263.2 kJ mol-O2−1 | (25) |
| 6Mn2O3 ↔ 4Mn3O4 + O2 ΔH850 = 193.9 kJ mol-O2−1 | (26) |
| 4Co3O4 ↔ 6CoO + O2 ΔH850 = 408.2 kJ mol-O2−1 | (27) |
The reactions between carbon/coal and Cu- or Mn-based OCs in the fuel reactor are exothermic. However, the reaction for Co-based OCs is endothermic. Therefore, a higher operation temperature is required for Co-based OCs than that for Cu- or Mn-based OCs. The endothermic nature of the reaction combined with the high cost of Co3O4 makes this type of OC unattractive. Thus, only Cu- and Mn-based materials are promising choices for CLOU.
The low melting point and agglomeration issues can be ignored for Cu-based OCs since no metallic Cu is involved in the loop of CLOU. Screening tests on 25 types of Cu-based materials were conducted in successive cycles in a thermogravimetric analyser (TGA), and it was found that CuO supported on MgAl2O4 and ZrO2 are the most promising owing to their stable reactivity.129 For Mn-based materials, natural manganese ore or the addition of Fe2O3, SiO2 and NiO were tested and mixing with Fe2O3 was observed to be the most effective way to increase their overall reactivity.130,131 A spinel perovskite structured material, CaMn0.875Ti0.125O3, was also developed as an OC for CLOU, although its performance was not as good as that of Cu-based materials.132 As discussed in Section 3, limited studies are focused on OU-BCLC and OU-BCLG, therefore more operational experience is necessary.
4.3. CO2 carrier (CC): LMs for BCaLC and BCaLG
Typical steam gasification of biomass suffers from two undesirable issues: CO2 from the water-gas shift reaction and the formation of tar. Thus, the requirements for CO2 carrier and biomass conversion catalysts include high CO2 capture capacity and selectivity as well as tar cracking ability. The use of CaO is also attractive since it may serve as both a CO2 sorbent and alkaline catalyst during biomass gasification.133 Since CaO can be regenerated in a calciner through calcium looping, BCaLG has been proposed.98Many CaO-based CO2 sorbents exhibit poor mechanical properties which cause them to degrade by attrition or elutriation. Furthermore, their sorption capacity decays rapidly over multiple cycles due to sintering.134,135 To enhance the recyclability of CaO, steam can be introduced into the regenerator.136 In addition to its use as an LM and catalyst, since limestone is extremely cheap and quite robust, CaO can also serve as a bed material or heat carrier to transfer heat from the regenerator (850–900 °C) to the gasifier (600–700 °C), where the exothermic carbonation reaction in the gasifier can also supply some heat.
Due to the high moisture content of biomass, the interaction of CaO with H2O is critical. It has been reported that H2O could significantly enhance the kinetics of the CaO carbonation process. Compared to the dry carbonation of CaO, wet carbonation is nine times faster.137 Thus, the presence of moisture in biomass may pose certain positive effects in BCaLC and BCaLG.
Another important feature of biomass is its low ash melting point. Due to the thermodynamics of CaO, carbonation is favoured at low temperatures, so the biomass reactor temperature is usually lower than 700 °C, which is beneficial to prevent biomass ash from melting.101 It has also been reported that the high sulphur content of coal may result in the sulphation of CaO which leads to deactivation. Calcium sulphate is inert compared to calcium carbonate and is difficult to remove during sorbent regeneration.138 This situation is less severe for biomass owing to its low sulphur content. It has even been reported that a CO2 sorbent prepared from rice husk ash and CaO hydration presented higher carbonation conversions than hydrated CaO or dry CaO during multiple tests. The reason for this was that the rice husk ash/CaO exhibited better anti-sintering behaviour compared to other sorbents.139
5. Challenges and opportunities
Although BCLPs have many potential benefits over the conventional biomass thermochemical methods and coal-fuelled CLPs, to date, this technology has not been widely commercialised. There are a number of issues associated with this technology that have to be considered and solved for its scale-up and commercialisation.5.1. Deactivation of looping materials during biomass conversion
The stability of LMs is a crucial issue. Chemical looping requires the characteristics of LMs to be stable (low attrition and thermal stability) after many cycles. There are many reasons that may cause the deactivation of LMs during BCLPs.5.2. Fouling due to unique biomass chemistry
Ash with a low melting point remaining in the fuel reactor can cause agglomeration and even defluidisation.59,60,77 By continuous removal of alkali metal laden bed particles from the system, a quasi-steady state of stable operation could be achieved by keeping the alkali metal content of the carrier particles below a critical level for stickiness and consequent agglomeration and breakdown of fluidisation. This suggested method of operation, which sacrifices the carrier material due to the withdrawal of alkali metals from the system, requires a low cost carrier such as ilmenite and excludes the more expensive specially designed oxygen carriers.
5.3. System complexity and scale
A higher O2/CO2 carrying capacity will reduce the required circulation rate. The carrying capacity depends on both the property of the reactive component and the extent of support. The effective carrying capacity could be influenced by the gas and solid residence time.17
5.4. Techno-economic evaluations
Currently, very few techno-economic evaluations of BCLPs are available in the open literature, but all of these studies demonstrated the reduced energy penalty of CLPs compared to other conventional processes combined with carbon capture units.Li simulated a BCLC plant using the Aspen Plus software.11 The system had a maximised efficiency of 38.1% with a CO2 capture efficiency of 99%. The electricity cost of BCLC was $95 per MW h, which was much lower than that of an integrated biomass gasification combined cycle, but still higher than the oxy-coal-combustion process with carbon capture ($66 per MW h).161 Recently, one new development has been the open release of the results from the UK's Energy Technologies Institute TESBIC programme.21 This project assessed the current technology readiness levels of BCLC as part of an overall assessment of 28 different combinations of CCS technology with biomass combustion or gasification, with co-firing also studied. Chemical looping was demonstrated to be highly valuable as a potential technology, and was one of the 8 technologies shortlisted for detailed modelling. The modelling produced process flow diagrams for each technology and compared chemical looping with combustion with amine scrubbing (both co-fired and dedicated biomass combustion), oxyfuel (co-fired and dedicated biomass combustion), co-fired carbonate looping, co-fired IGCC and dedicated biomass IGCC. Potential issues were identified for each technology, which for BCLC were potential loss in reactivity of the OCs and the complexity of the dual bed operation. Importantly, relative to other technologies, BCLC was ranked as having a low capital cost when capture was added as well as good efficiency. It was noted in the study that it is challenging to compare technologies at a high level of technology readiness with that at a lower level. The overall findings of the study indicated that given the range of uncertainty in capital costs it is unwise to pick a favourite technology for long-term exploitation, but that continued research and development of BCLC is certainly justified. Thus, a further study was commissioned to reduce the overall error bounds for high temperature solid looping technologies.
Aghabararnejad et al.162 compared BCLG to conventional biomass gasification with air and found that BCLG can produce reformer gas with a higher calorific value. The use of steam can enhance the purity of H2, however, steam gasification is endothermic. For comparison, a conventional gasification unit with pure oxygen (CGPO) and a BCLG system were modelled with Aspen Plus in a separate study.162 A 7 MWth BCLG unit was simulated to treat biomass (86 t d−1). A bubbling-bed gasifier (fuel reactor) and a fast fluidised bed oxidiser (air reactor) were designed for the study. Co3O4(8%)/Al2O3 (44.6 kg s−1 circulation rate) was selected as the OC. The total capital investment of the BCLG unit was $3.4 M higher than that of the CGPO, whereas annual production costs of the CGPO and CLG units were found to be $1.9 M and $1.32 M, respectively. The main difference between the operating costs of the units is due to the cost of raw materials. The pure O2 supply contributes most to the overall operating costs for CGPO. On the other hand, the OCs can be recycled due to their long lifetimes, which reduces replacement costs.
A techno-economic evaluation of H2 (up to 200 MWth) and power (400–500 MWe) co-generation from a sawdust-fired BCCLP (with an ilmenite OC) was also conducted.88 The BCCLP concept was compared to various benchmark cases with or without carbon capture. Selexol®-based gas–liquid absorption and syngas chemical looping were selected as the benchmark cases for carbon capture and their energy penalty was found to be 9.2 and 8 net electricity percentage points, respectively, in comparison to 3.5% for BCCLP. The operational and maintenance cost of BCCLP is higher than the benchmark cases, which is mainly due to the cost incurred for regenerating OCs. However, the cost of electricity of BCCLP is lower than gas–liquid design (about 3.7%) and higher than the syngas-based chemical looping case (5.7%). Chemical looping not only achieves a higher energy efficiency (∼42% net efficiency) but also nearly complete CO2 capture (>99%). Moreover, the overall plant energy efficiency (power + hydrogen output) can be increased by 7 net efficiency points when the hydrogen output is 200 MWth.
The application of CaO-based CaLG using coal can be traced back to the 1970s, where it was tested successfully in a pilot plant and proved to be economically feasible.98 However, BCaLG using biomass took a lot longer to be trialled. BCaLG based on CaO on the scale of 100 kW, 120 kW, and 8 MW was reported in 2009.108,109 In the 100 kW and 120 kW tests, the concentration of H2 in the produced gas was as high as 75%. Also, the 8 MW test proved the larger-scale viability of this idea.108,109
6. Conclusions and perspectives
Biomass is an important energy source and its further development will lessen our dependence on fossil fuels. Considering its carbon-neutral nature, significant environmental benefits are expected, since the world is seeking clean, renewable energy solutions to reduce net GHG emissions. However, owing to its low energy density, high moisture content, complex ash composition and highly distributed resource, biomass is often less favoured in conventional thermochemical processes compared to fossil fuels. Conventional energy conversion systems are generally not directly integrated with CO2 capture. Therefore, the search for a conversion system that can provide high efficiency, product flexibility (ranging from electricity to chemicals), in situ carbon capture, and scalability (small to large) has been intense. As discussed in this review, biomass-based chemical looping technologies have gained significant attention and have great potential to provide a sustainable pathway towards decarbonised energy and materials production.How to get there? The good, the bad, and the future of biomass-based CLPs includes:
The good: biomass-based chemical looping is promising due to its low exergy loss and capacity for inherent CO2 separation. When biomass is directly used in chemical looping, no additional energy is required for CO2 capture (energy is only required for compression for transport), the overall exergy loss is minimised, and if combined with appropriate carbon storage, a net negative carbon balance can be achieved. Moreover, the relatively high quantity of volatile matter, and low sulphur and ash content of biomass will improve the operation of a chemical looping process compared to coal utilisation. Some looping materials allow chemical looping oxygen uncoupling (CLOU) to be realised with consequently faster biomass conversion rates. Instead of heat or power, syngas, hydrogen or even carbon-based chemicals can be produced through biomass-based chemical looping gasification (CLG) or calcium looping gasification (CaLG). This flexibility in product type distinguishes biomass-based chemical looping processes (BCLPs) from other renewable energy sources such as solar or wind. A number of studies have already demonstrated the recyclability of looping materials and recent techno-economic assessments suggest a net reduction in energy penalty. Thus, biomass-based chemical looping has significant potential to offer a sustainable and efficient pathway to utilise biomass resources in an efficient manner, coupled with the potential to effectively remove CO2 (net) from the atmosphere.
The bad: although biomass-based chemical looping processes have many advantages compared to traditional biomass utilisation methods, they also share some challenges such as looping material deactivation, high solid recirculation rate, and the requirement for separation of looping materials and biomass ash. Therefore, significant efforts are currently devoted to solve these problems for the ultimate goal of commercializing this technology. Continuous efforts are in progress to determine the details of its reaction mechanisms, kinetics, mass transfer and other operational challenges. Once these have been identified and fully addressed, further overall systems integration studies are required to minimise exergy loss within the overall system, while improving the economics and environmental sustainability of the developed technology.
The future: similarly to many other disruptive technologies, the large-scale implementation of biomass-based chemical looping technologies will not be easy, but the potential gain should outweigh the challenge. There are a number of pilot-scale demonstrations of chemical looping processes, which will be important bases for the future of biomass-based chemical looping processes. Despite the fact that results in pilots may not be directly translatable to commercial-sized units, the understanding, experience, and know-how gained from fundamental and small-scale research will be central for the development of biomass-based chemical looping technologies.
Some future thoughts/perspectives on BCLPs are as follows:
(a) Development of biomass gasification processes for syngas generation and chemicals/liquid fuels production should focus on process intensification.163 Process intensification should allow for the conversion of biomass to high quality syngas with an appropriate H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio in a single step without the use of molecular oxygen and capital-intensive units including tar reformer, water gas shift reactors, and air separation units. Such high-quality syngas generation would permit downstream processing to produce chemicals/liquid fuels without requiring syngas re-conditioning while reducing the capital and operating costs associated with acid gas (CO2 and sulphur) removal.
CO ratio in a single step without the use of molecular oxygen and capital-intensive units including tar reformer, water gas shift reactors, and air separation units. Such high-quality syngas generation would permit downstream processing to produce chemicals/liquid fuels without requiring syngas re-conditioning while reducing the capital and operating costs associated with acid gas (CO2 and sulphur) removal.
(b) Holistic evaluation of the system operating pressure is crucial for ensuring the cost-competitiveness of biomass-based chemical looping systems. A higher operating pressure increases the local concentration of the reactants on the LM, thereby enhancing the kinetics of biomass conversion. This helps to reduce the reactor size and compression costs associated with the syngas product while increasing the cost of construction materials and re-oxidation air compression costs. Optimization of operating pressures for chemical looping reactors using multi-phase flow reactor engineering and techno-economic considerations and corresponding pilot scale experimental verification is necessary.
(c) Research towards the enhancement of the multifunctional nature of LM, while sustaining reactivity and structural integrity for thousands of redox cycles is crucial. An example of multifunctional enhancement is the development of an LM that can gasify biomass char and crack biomass tar to syngas in a single reactor using new dopants and support materials. The rationale for the choice of dopants and support materials for LM should be driven by a deep scientific understanding of material properties which is derived using tools such as DFT/molecular modelling aided by experimental verification. The developed multifunctional LMs should be screened for their lifecycle based impact and cost of production.
(d) Linkage of industrial processes with BCLP, most likely via BCLC, BCLG, and bottom-up redesign of current “state of the art” processes. For example, there is significant synergy between Ca-looping and cement manufacturing. Collaboration between the power and chemical industries for pilot scale demonstrations will greatly benefit the commercialization of BCLP.
(e) In addition to screening for holistic economic advantages for one specific biomass feedstock, operational flexibility in the variation of feedstock composition is required. This versatility would allow for fast response to the changes in supply and demand of the feedstock market.
(f) BCLP is also a potential strategy to obtain energy from biomass waste (such as woody waste, municipal waste or dry sludge from wastewater treatment plants) with a net carbon negative balance. CO2 utilization strategies, which have the potential to enhance syngas yields, should be implemented.164 A focus on the economic conversion of biomass waste to high value chemical products and liquid fuels would accelerate the large-scale commercial deployment of BCLP.
Fundamental knowledge gained from these studies should be shared with those researching other biomass conversion and chemical looping technologies in order to accelerate the realisation of BECCS as a crucial technology in the continued fight against climate change.
Glossary
| ASTM | American Society for Testing and Materials |
| BECCS | Bioenergy with carbon capture and storage |
| BCaLC | Biomass-based calcium looping combustion |
| BCaLG | Biomass-based calcium looping gasification |
| BCCLP | Biomass-based co-production chemical looping process |
| BCLC | Biomass-based chemical looping combustion |
| BCLG | Biomass-based chemical looping gasification |
| BCLP | Biomass-based chemical looping process |
| CCS | Carbon capture and storage |
| CC | CO2 carrier |
| CGPO | Conventional gasification unit with pure oxygen |
| CaLC | Calcium looping combustion |
| CaLG | Calcium looping gasification |
| CLC | Chemical looping combustion |
| CLG | Chemical looping gasification |
| CLOU | Chemical looping oxygen uncoupling |
| CLP | Chemical looping process |
| CLR | Chemical looping reforming |
| GHG | Greenhouse gas |
| IGCC | Integrated gasification combined cycle |
| iG-CLC | In situ gasification chemical looping combustion |
| IPCC | Intergovernmental panel on climate change |
| LHV | Low heating value |
| LM | Looping material |
| OC | Oxygen carrier |
| OU-BCLC | Oxygen uncoupling biomass-based CLC |
| OU-BCLG | Oxygen uncoupling biomass-based CLG |
| SE-BCLG | Sorption enhanced BCLG |
| SNG | Synthetic natural gas |
| TESBIC | Techno-economic study of biomass to power with integrated CO2 capture |
| PAH | Polycyclic aromatic hydrocarbon |
| TGA | Thermogravimetric analyser |
| UNFCCC | United Nations Framework Convention on Climate Change |
Acknowledgements
This work was supported by the National Recruitment Program of Global Youth Experts (The National Youth 1000 – Talent Program) of China (grant number: 20151710227); Tsinghua University Initiative Scientific Research Program (grant number: 20161080094); United States National Science Foundation (CBET 1336567 and CBET 1231393). The helpful assistance of Arun K. Vuppaladadiyam for preparing Fig. 1 is gratefully acknowledged. PSF thanks the EPSRC for funding under grant number EP/P026214/1 (UK CCS Research Centre).References
- S. Manabe and R. T. Wetherald, J. Atmos. Sci., 1980, 37, 99–118 CrossRef.
- A. Goeppert, M. Czaun, J.-P. Jones, G. S. Prakash and G. A. Olah, Chem. Soc. Rev., 2014, 43, 7995–8048 RSC.
- BP, BP Statistical Review of World Energy June 2016, http://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html.
- CO 2 Emissions, The World Bank, 2016.
- J. Rogelj and R. Knutti, Nat. Geosci., 2016, 9, 187–189 CrossRef CAS.
- J. Adanez, A. Abad, F. Garcia-Labiano, P. Gayan and F. Luis, Prog. Energy Combust. Sci., 2012, 38, 215–282 CrossRef CAS.
- M. Zhao, A. I. Minett and A. T. Harris, Energy Environ. Sci., 2013, 6, 25–40 CAS.
- R. D. Perlack, L. M. Eaton, A. F. Turhollow Jr, M. H. Langholtz, C. C. Brandt, M. E. Downing, R. L. Graham, L. L. Wright, J. M. Kavkewitz and A. M. Shamey, US billion-ton update: biomass supply for a bioenergy and bioproducts industry, Oak Ridge Naitonal Lab, Oak Ridge, TN, 2011 Search PubMed.
- P. Lamers, R. Hoefnagels, M. Junginger, C. Hamelinck and A. Faaij, GCB Bioenergy, 2015, 7, 618–634 CrossRef.
- IPCC, Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, New York, 2013, vol. 1.
- F. Li, L. Zeng and L.-S. Fan, Fuel, 2010, 89, 3773–3784 CrossRef CAS.
- N. Kobayashi and L.-S. Fan, Biomass Bioenergy, 2011, 35, 1252–1262 CrossRef CAS.
- M. S. Mettler, D. G. Vlachos and P. J. Dauenhauer, Energy Environ. Sci., 2012, 5, 7797–7809 CAS.
- L.-S. Fan, L. Zeng, W. Wang and S. Luo, Energy Environ. Sci., 2012, 5, 7254–7280 CAS.
- L.-S. Fan, L. Zeng and S. Luo, AIChE J., 2015, 61, 2–22 CrossRef CAS.
- S. Luo, L. Zeng and L.-S. Fan, Annu. Rev. Chem. Biomol. Eng., 2015, 53–75 CrossRef CAS PubMed.
- L.-S. Fan, Chemical Looping Systems for Fossil Fuel Conversions, John Wiley and Sons, New York, 2010 Search PubMed.
- E. R. Gilliland, Production of industrial gas comprising carbon monoxide and hydrogen, US Pat., 2671721, 1954 Search PubMed.
- M. Ishida, D. Zheng and T. Akehata, Energy, 1987, 12, 147–154 CrossRef CAS.
- B. Moghtaderi, Energy Fuels, 2011, 26, 15–40 CrossRef.
- A. Bhave, R. H. S. Taylor, P. Fennell, W. R. Livingston, N. Shah, N. M. Dowell, J. Dennis, M. Kraft, M. Pourkashanian, M. Insa, J. Jones, N. Burdett, A. Bauen, C. Beal, A. Smallbone and J. Akroyd, Appl. Energy, 2017, 190, 481–489 CrossRef CAS.
- S. V. Vassilev, D. Baxter, L. K. Andersen and C. G. Vassileva, Fuel, 2013, 105, 40–76 CrossRef CAS.
- S. V. Vassilev, D. Baxter, L. K. Andersen, C. G. Vassileva and T. J. Morgan, Fuel, 2012, 94, 1–33 CrossRef CAS.
- S. V. Vassilev, D. Baxter and C. G. Vassileva, Fuel, 2014, 117, 152–183 CrossRef CAS.
- M. Tang, L. Xu and M. Fan, Appl. Energy, 2015, 151, 143–156 CrossRef CAS.
- F. He, H. Li and Z. Zhao, Int. J. Chem. Eng., 2009, 2009, 710515, DOI:10.1155/2009/710515.
- Q. Imtiaz, D. Hosseini and C. R. Müller, Energy Technol., 2013, 1, 633–647 CrossRef CAS.
- P. Markström, C. Linderholm and A. Lyngfelt, Int. J. Greenhouse Gas Control, 2013, 15, 150–162 CrossRef.
- A. Lyngfelt, Appl. Energy, 2014, 113, 1869–1873 CrossRef CAS.
- P. Basu, Biomass gasification and pyrolysis: Practical design and theory, Academic Press, 2010 Search PubMed.
- D. Gera, Biofules and bioenergy, Encylopedia of chemical processing, CRC Press, 2005 Search PubMed.
- I. E. A. (IEA), Technology Roadmap Bioenergy for Heat and Power, 2012.
- A. Demirbas, Energy Sources, 2006, 28, 779–792 CrossRef.
- R. Luque, Energy Environ. Sci., 2010, 3, 254–257 CAS.
- H. Tadesse and R. Luque, Energy Environ. Sci., 2011, 4, 3913–3929 CAS.
- P. Sannigrahi, A. J. Ragauskas and G. A. Tuskan, Biofuels, Bioprod. Biorefin., 2010, 4, 209–226 CrossRef CAS.
- D. Shen and S. Gu, Bioresour. Technol., 2009, 100, 6496–6504 CrossRef CAS PubMed.
- R. Rinaldi and F. Schüth, Energy Environ. Sci., 2009, 2, 610–626 CAS.
- P. McKendry, Bioresour. Technol., 2002, 83, 37–46 CrossRef CAS PubMed.
- S. V. Vassilev, C. G. Vassileva and V. S. Vassilev, Fuel, 2015, 158, 330–350 CrossRef CAS.
- S. V. Vassilev, D. Baxter, L. K. Andersen and C. G. Vassileva, Fuel, 2010, 89, 913–933 CrossRef CAS.
- C. Linderhom and A. Lynfelt, Chemical-looping combustion of solid fuels, in Calcium and Chemical Looping Technology for Power Generation and Carbon Dioxide (CO2) Capture, ed. P. Fennell and B. Anthony, 2015, ch. 14, pp. 299–326 Search PubMed.
- D. Das and T. N. Veziroǧlu, Int. J. Hydrogen Energy, 2001, 26, 13–28 CrossRef CAS.
- E. Kırtay, Energy Convers. Manage., 2011, 52, 1778–1789 CrossRef.
- W. Gerbens-Leenes, A. Y. Hoekstra and T. H. van der Meer, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10219–10223 CrossRef CAS PubMed.
- USDOE, Post-combustion carbon capture research, http://https://energy.gov/fe/science-innovation/carbon-capture-and-storage-research/carbon-capture-rd/post-combustion-carbon, Department Of Energy, 2012.
- A. Nandy, C. Loha, S. Gu, P. Sarkar, M. K. Karmakar and P. K. Chatterjee, Renewable Sustainable Energy Rev., 2016, 59, 597–619 CrossRef.
- T. Nussbaumer, Energy Fuels, 2003, 17, 1510–1521 CrossRef CAS.
- B. Jenkins, L. Baxter and T. Miles, Fuel Process. Technol., 1998, 54, 17–46 CrossRef CAS.
- Y. Niu, H. Tan and S. e. Hui, Prog. Energy Combust. Sci., 2016, 52, 1–61 CrossRef.
- J. Han and H. Kim, Renewable Sustainable Energy Rev., 2008, 12, 397–416 CrossRef CAS.
- H. Leion, T. Mattisson and A. Lyngfelt, CO2capture from direct combustion of solid fuels with chemical-looping combustion, The Proceedings of the 33rd International Technical Conference on Coal Utilization & Fuel Systems, 2008.
- L. Shen, J. Wu, J. Xiao, Q. Song and R. Xiao, Energy Fuels, 2009, 23, 2498–2505 CrossRef CAS.
- Y. Cao and W.-P. Pan, Energy Fuels, 2006, 20, 1836–1844 CrossRef CAS.
- T. J. Kotas, The Exergy Method of Thermal Plant Analysis, Butterworth Publishers, London, UK, 1985 Search PubMed.
- T. Mattisson, M. Johansson and A. Lyngfelt, CO2capture from coal combustion using chemical-looping combustion—reactivity investigation of Fe, Ni and Mn based oxygen carriers using syngas, Proceedings of the Clearwater Coal Conference, 2006.
- T. Mattisson, A. Lyngfelt and H. Leion, Int. J. Greenhouse Gas Control, 2009, 3, 11–19 CrossRef CAS.
- T. Mendiara, A. Abad, L. de Diego, F. García-Labiano, P. Gayán and J. Adánez, Int. J. Greenhouse Gas Control, 2013, 19, 322–330 CrossRef CAS.
- X. Niu, L. Shen, H. Gu, S. Jiang and J. Xiao, Chem. Eng. J., 2015, 268, 236–244 CrossRef CAS.
- X. Niu, L. Shen, H. Gu, T. Song and J. Xiao, Chem. Eng. J., 2015, 260, 631–641 CrossRef CAS.
- A. Sarvaramini and F. Larachi, Fuel, 2014, 116, 158–167 CrossRef CAS.
- A. Sarvaramini and F. Larachi, Energy Fuels, 2014, 28, 1983–1991 CrossRef CAS.
- R. J. Evans and T. A. Milne, Energy Fuels, 1987, 1, 311–319 CrossRef CAS.
- A. Cuadrat, A. Abad, F. García-Labiano, P. Gayán, L. De Diego and J. Adánez, Int. J. Greenhouse Gas Control, 2011, 5, 1630–1642 CrossRef CAS.
- A. Lyngfelt and T. Mattisson, Trestegsfo rbranning for avskiljning av koldioxid, Swedish Pat., SE 0500249-8, 2005 Search PubMed.
- I. Adánez-Rubio, A. Abad, P. Gayán, L. De Diego, F. García-Labiano and J. Adánez, Fuel Process. Technol., 2014, 124, 104–114 CrossRef.
- T. Mendiara, I. Adánez-Rubio, P. Gayán, A. Abad, L. de Diego, F. García-Labiano and J. Adánez, Energy Technol., 2016, 4, 1130–1136 CrossRef CAS.
- A. Corcoran, J. Marinkovic, F. Lind, H. Thunman, P. Knutsson and M. Seemann, Energy Fuels, 2014, 28, 7672–7679 CrossRef CAS.
- H. Thunman, F. Lind, C. Breitholtz, N. Berguerand and M. Seemann, Fuel, 2013, 113, 300–309 CrossRef CAS.
- H. Gu, L. Shen, J. Xiao, S. Zhang and T. Song, Energy Fuels, 2011, 25, 446–455 CrossRef CAS.
- G. Wei, F. He, Z. Huang, A. Zheng, K. Zhao and H. Li, Energy Fuels, 2015, 29, 233–241 CrossRef CAS.
- S. Huseyin, G.-q. Wei, H.-b. Li, H. Fang and Z. Huang, J. Fuel Chem. Technol., 2014, 42, 922–931 CrossRef CAS.
- M. Virginie, J. Adánez, C. Courson, L. De Diego, F. García-Labiano, D. Niznansky, A. Kiennemann, P. Gayán and A. Abad, Appl. Catal., B, 2012, 121, 214–222 CrossRef.
- Z. Huang, F. He, Y. Feng, R. Liu, K. Zhao, A. Zheng, S. Chang, Z. Zhao and H. Li, Int. J. Hydrogen Energy, 2013, 38, 14568–14575 CrossRef CAS.
- Z. Huang, F. He, Y. Feng, K. Zhao, A. Zheng, S. Chang and H. Li, Bioresour. Technol., 2013, 140, 138–145 CrossRef CAS PubMed.
- Z. Huang, F. He, K. Zhao, Y. Feng, A. Zheng, S. Chang, Z. Zhao and H. Li, J. Therm. Anal. Calorim., 2014, 116, 1315–1324 CrossRef CAS.
- H. Ge, W. Guo, L. Shen, T. Song and J. Xiao, Chem. Eng. J., 2016, 286, 174–183 CrossRef CAS.
- F. García-Labiano, E. García-Díez, L. De Diego, A. Serrano, A. Abad, P. Gayán, J. Adánez and J. Ruíz, Fuel Process. Technol., 2015, 137, 24–30 CrossRef.
- A. Lea-Langton, R. M. Zin, V. Dupont and M. V. Twigg, Int. J. Hydrogen Energy, 2012, 37, 2037–2043 CrossRef CAS.
- G. Huijun, S. Laihong, F. Fei and J. Shouxi, Appl. Therm. Eng., 2015, 85, 52–60 CrossRef.
- L. Guo, H. Zhao and C. Zheng, Waste Biomass Valorization, 2015, 6, 81–89 CrossRef CAS.
- G. Wei, F. He, Z. Zhao, Z. Huang, A. Zheng, K. Zhao and H. Li, Int. J. Hydrogen Energy, 2015, 40, 16021–16032 CrossRef CAS.
- J. Ran, F. Fu, C. Qin, P. Zhang, L. Yang, W. Wang and L. Yang, BioResources, 2016, 11, 2109–2123 CrossRef CAS.
- H. Zhao, L. Guo and X. Zou, Appl. Energy, 2015, 157, 408–415 CrossRef CAS.
- Z. Huang, F. He, H. Zhu, D. Chen, K. Zhao, G. Wei, Y. Feng, A. Zheng, Z. Zhao and H. Li, Appl. Energy, 2015, 157, 546–553 CrossRef CAS.
- Z. Huang, Y. Zhang, J. Fu, L. Yu, M. Chen, S. Liu, F. He, D. Chen, G. Wei, K. Zhao, A. Zheng, Z. Zhao and H. Li, Int. J. Hydrogen Energy, 2016, 41, 17871–17883 CrossRef CAS.
- H. Ge, W. Guo, L. Shen, T. Song and J. Xiao, Chem. Eng. J., 2016, 286, 689–700 CrossRef CAS.
- C.-C. Cormos, Fuel Process. Technol., 2015, 137, 16–23 CrossRef CAS.
- S. G. Gopaul, A. Dutta and R. Clemmer, Int. J. Hydrogen Energy, 2014, 39, 5804–5817 CrossRef CAS.
- L. Yan, B. He, X. Pei, C. Wang, Z. Duan, J. Song and X. Li, Int. J. Hydrogen Energy, 2014, 39, 17540–17553 CrossRef CAS.
- M. Rydén and M. Arjmand, Int. J. Hydrogen Energy, 2012, 37, 4843–4854 CrossRef.
- J. C. Abanades, M. n. Alonso and N. Rodríguez, Ind. Eng. Chem. Res., 2011, 50, 6972–6981 CrossRef CAS.
- M. N. Alonso, N. Rodríguez, B. N. González, B. Arias and J. C. Abanades, Ind. Eng. Chem. Res., 2011, 50, 6982–6989 CrossRef CAS.
- M. Alonso, M. Diego, C. Pérez, J. Chamberlain and J. Abanades, Int. J. Greenhouse Gas Control, 2014, 29, 142–152 CrossRef CAS.
- D. C. Ozcan, M. n. Alonso, H. Ahn, J. C. Abanades and S. Brandani, Ind. Eng. Chem. Res., 2014, 53, 10721–10733 CrossRef CAS.
- M. R. Stonor, T. E. Ferguson, J. G. Chen and A.-H. A. Park, Energy Environ. Sci., 2015, 8, 1702–1706 CAS.
- F. Yin, K. Shah, C. Zhou, P. Tremain, J. Yu, E. Doroodchi and B. Moghtaderi, Energy Fuels, 2016, 30, 1730–1740 CrossRef CAS.
- J. Udomsirichakorn and P. A. Salam, Renewable Sustainable Energy Rev., 2014, 30, 565–579 CrossRef CAS.
- B. Moghtaderi, J. Zanganeh, K. Shah and H. Wu, Energy Fuels, 2012, 26, 2046–2057 CrossRef CAS.
- B. Acharya, A. Dutta and P. Basu, Energy Fuels, 2009, 23, 5077–5083 CrossRef CAS.
- H. Gu, G. Song, J. Xiao, H. Zhao and L. Shen, Energy Fuels, 2013, 27, 4695–4704 CrossRef CAS.
- L. Wei, S. Xu, J. Liu, C. Liu and S. Liu, Energy Fuels, 2008, 22, 1997–2004 CrossRef CAS.
- B. Dou, Y. Song, C. Wang, H. Chen, M. Yang and Y. Xu, Appl. Energy, 2014, 130, 342–349 CrossRef CAS.
- P. Pimenidou, G. Rickett, V. Dupont and M. Twigg, Bioresour. Technol., 2010, 101, 6389–6397 CrossRef CAS PubMed.
- P. Pimenidou, G. Rickett, V. Dupont and M. V. Twigg, Bioresour. Technol., 2010, 101, 9279–9286 CrossRef CAS PubMed.
- T. Udomchoke, S. Wongsakulphasatch, W. Kiatkittipong, A. Arpornwichanop, W. Khaodee, J. Powell, J. Gong and S. Assabumrungrat, Chem. Eng. J., 2016, 303, 338–347 CrossRef CAS.
- J. Udomsirichakorn, P. Basu, P. A. Salam and B. Acharya, Fuel Process. Technol., 2014, 127, 7–12 CrossRef CAS.
- S. Koppatz, C. Pfeifer, R. Rauch, H. Hofbauer, T. Marquard-Moellenstedt and M. Specht, Fuel Process. Technol., 2009, 90, 914–921 CrossRef CAS.
- G. Soukup, C. Pfeifer, A. Kreuzeder and H. Hofbauer, Chem. Eng. Technol., 2009, 32, 348–354 CrossRef CAS.
- L. F. de Diego, M. Ortiz, F. García-Labiano, J. Adánez, A. Abad and P. Gayán, J. Power Sources, 2009, 192, 27–34 CrossRef CAS.
- A. Lyngfelt, P. Fennell and B. Anthony, Calcium and Chemical Looping Technology for Power Generation and Carbon Dioxide (CO2) Capture, 2015, pp. 221–254 Search PubMed.
- J. Adánez, C. Dueso, L. F. de Diego, F. García-Labiano, P. Gayán and A. Abad, Ind. Eng. Chem. Res., 2009, 48, 2509–2518 CrossRef.
- A. Lyngfelt, Oil Gas Sci. Technol., 2011, 66, 161–172 CrossRef CAS.
- M. Ishida and H. Jin, J. Chem. Eng. Jpn., 1994, 27, 296–301 CrossRef CAS.
- F. García-Labiano, L. F. de Diego, P. Gayán, J. Adánez, A. Abad and C. Dueso, Ind. Eng. Chem. Res., 2009, 48, 2499–2508 CrossRef.
- J. Adanez, L. F. de Diego, F. García-Labiano, P. Gayán, A. Abad and J. Palacios, Energy Fuels, 2004, 18, 371–377 CrossRef CAS.
- A. Abad, J. Adánez, F. García-Labiano, F. Luis, P. Gayán and J. Celaya, Chem. Eng. Sci., 2007, 62, 533–549 CrossRef CAS.
- P. Cho, T. Mattisson and A. Lyngfelt, Ind. Eng. Chem. Res., 2005, 44, 668–676 CrossRef CAS.
- E. Jerndal, T. Mattisson and A. Lyngfelt, Chem. Eng. Res. Des., 2006, 84, 795–806 CrossRef CAS.
- M. Rydén, E. Cleverstam, M. Johansson, A. Lyngfelt and T. Mattisson, AIChE J., 2010, 56, 2211–2220 Search PubMed.
- C. Forero, P. Gayán, F. García-Labiano, L. De Diego, A. Abad and J. Adánez, Int. J. Greenhouse Gas Control, 2010, 4, 762–770 CrossRef CAS.
- S. Chuang, J. Dennis, A. Hayhurst and S. Scott, Combust. Flame, 2008, 154, 109–121 CrossRef CAS.
- Q. Zafar, T. Mattisson and B. Gevert, Energy Fuels, 2006, 20, 34–44 CrossRef CAS.
- H. Tian, T. Simonyi, J. Poston and R. Siriwardane, Ind. Eng. Chem. Res., 2009, 48, 8418–8430 CrossRef CAS.
- M. Johansson, T. Mattisson and A. Lyngfelt, Chem. Eng. Res. Des., 2006, 84, 807–818 CrossRef CAS.
- H. Jin, T. Okamoto and M. Ishida, Energy Fuels, 1998, 12, 1272–1277 CrossRef CAS.
- T. Mattisson, A. Järdnäs and A. Lyngfelt, Energy Fuels, 2003, 17, 643–651 CrossRef CAS.
- K. Salazar and M. K. McNutt, Mineral commodity summaries, US Geological Survey, Reston, VA, 2012 Search PubMed.
- I. Adanez-Rubio, P. Gayán, F. García-Labiano, F. Luis, J. Adánez and A. Abad, Energy Procedia, 2011, 4, 417–424 CrossRef CAS.
- A. Shulman, E. Cleverstam, T. Mattisson and A. Lyngfelt, Energy Fuels, 2009, 23, 5269–5275 CrossRef CAS.
- M. Rydén, A. Lyngfelt and T. Mattisson, Energy Procedia, 2011, 4, 341–348 CrossRef.
- H. Leion, Y. Larring, E. Bakken, R. Bredesen, T. Mattisson and A. Lyngfelt, Energy Fuels, 2009, 23, 5276–5283 CrossRef CAS.
- L. Han, Q. Wang, Y. Yang, C. Yu, M. Fang and Z. Luo, Int. J. Hydrogen Energy, 2011, 36, 4820–4829 CrossRef CAS.
- J. Wang, V. Manovic, Y. Wu and E. J. Anthony, Appl. Energy, 2010, 87, 1453–1458 CrossRef CAS.
- P. Sun, J. Grace, C. Lim and E. Anthony, AIChE J., 2007, 53, 2432–2442 CrossRef CAS.
- F. Donat, N. H. Florin, E. J. Anthony and P. S. Fennell, Environ. Sci. Technol., 2012, 46, 1262–1269 CrossRef CAS PubMed.
- V. Nikulshina, M. E. Gálvez and A. Steinfeld, Chem. Eng. J., 2007, 129, 75–83 CrossRef CAS.
- D. Y. Lu, R. W. Hughes and E. J. Anthony, Fuel Process. Technol., 2008, 89, 1386–1395 CrossRef CAS.
- Y. Li, C. Zhao, Q. Ren, L. Duan, H. Chen and X. Chen, Fuel Process. Technol., 2009, 90, 825–834 CrossRef CAS.
- T. Mattisson, M. Johansson and A. Lyngfelt, Fuel, 2006, 85, 736–747 CrossRef CAS.
- P. Cho, T. Mattisson and A. Lyngfelt, Fuel, 2004, 83, 1215–1225 CrossRef CAS.
- T. Mattisson, M. Johansson, E. Jerndal and A. Lyngfelt, Can. J. Chem. Eng., 2008, 86, 756–767 CrossRef CAS.
- P. Gayán, F. Luis, F. García-Labiano, J. Adánez, A. Abad and C. Dueso, Fuel, 2008, 87, 2641–2650 CrossRef.
- P. Gayán, C. Dueso, A. Abad, J. Adanez, F. Luis and F. García-Labiano, Fuel, 2009, 88, 1016–1023 CrossRef.
- I. Iliuta, R. Tahoces, G. S. Patience, S. Rifflart and F. Luck, AIChE J., 2010, 56, 1063–1079 CAS.
- L. F. de Diego, P. Gayán, F. García-Labiano, J. Celaya, A. Abad and J. Adánez, Energy Fuels, 2005, 19, 1850–1856 CrossRef CAS.
- W. H. Calkins, Fuel, 1994, 73, 475–484 CrossRef CAS.
- M. Keller, M. Arjmand, H. Leion and T. Mattisson, Chem. Eng. Res. Des., 2014, 92, 1753–1770 CrossRef CAS.
- C.-L. Lin, J.-H. Kuo, M.-Y. Wey, S.-H. Chang and K.-S. Wang, Powder Technol., 2009, 189, 57–63 CrossRef CAS.
- M. M. Azis, H. Leion, E. Jerndal, B. M. Steenari, T. Mattisson and A. Lyngfelt, Chem. Eng. Technol., 2013, 36, 1460–1468 CrossRef CAS.
- H. Gu, L. Shen, Z. Zhong, Y. Zhou, W. Liu, X. Niu, H. Ge, S. Jiang and L. Wang, Chem. Eng. J., 2015, 277, 70–78 CrossRef CAS.
- C. Wu, L. Wang, P. T. Williams, J. Shi and J. Huang, Appl. Catal., B, 2011, 108, 6–13 CrossRef.
- T. Furusawa, Y. Miura, Y. Kori, M. Sato and N. Suzuki, Catal. Commun., 2009, 10, 552–556 CrossRef CAS.
- S. Luo, A. Majumder, E. Chung, D. Xu, S. Bayham, Z. Sun, L. Zeng and L.-S. Fan, Ind. Eng. Chem. Res., 2013, 52, 14116–14124 CrossRef CAS.
- E. B.-H. Matthew, F. Nick and S. F. Paul, Environ. Res. Lett., 2016, 11, 115001 CrossRef.
- A. Demirbas, Prog. Energy Combust. Sci., 2004, 30, 219–230 CrossRef CAS.
- ASTM, Standard test method for ash in wood, D-1102-84 American Society for Testing and Materials, Philadelphia, PA, 2003.
- R. Saidur, E. Abdelaziz, A. Demirbas, M. Hossain and S. Mekhilef, Renewable Sustainable Energy Rev., 2011, 15, 2262–2289 CrossRef CAS.
- A. A. Rentizelas, A. J. Tolis and I. P. Tatsiopoulos, Renewable Sustainable Energy Rev., 2009, 13, 887–894 CrossRef.
- U. Drescher and D. Brüggemann, Appl. Therm. Eng., 2007, 27, 223–228 CrossRef CAS.
- J. S. Rhodes and D. W. Keith, Biomass Bioenergy, 2005, 29, 440–450 CrossRef.
- M. Aghabararnejad, G. S. Patience and J. Chaouki, Chem. Eng. Technol., 2015, 38, 867–878 CrossRef CAS.
- L.-S. Fan, Chemical Looping Partial Oxidation Gasification: Reforming, and Chemical Syntheses, Cambridge University Press, London, 2017 Search PubMed.
- M. Kathe, A. Empfield, P. Sandvik, C. Fryer, Y. Zhang, E. Blair and L.-S. Fan, Energy Environ. Sci., 2017 10.1039/C6EE03701A.
Footnote |
| † Equal contributions. |
| This journal is © The Royal Society of Chemistry 2017 |