Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optical limiters with improved performance based on nanoconjugates of thiol substituted phthalocyanine with CdSe quantum dots and Ag nanoparticles†
David O.
Oluwole
 a,
Alexey V.
Yagodin
a,
Alexey V.
Yagodin
 bc,
Jonathan
Britton
a,
Alexander G.
Martynov
bc,
Jonathan
Britton
a,
Alexander G.
Martynov
 c,
Yulia G.
Gorbunova
c,
Yulia G.
Gorbunova
 *cd,
Aslan Yu.
Tsivadze
cd and
Tebello
Nyokong
*cd,
Aslan Yu.
Tsivadze
cd and
Tebello
Nyokong
 *a
*a
aDepartment of Chemistry, Rhodes University, Grahamstown 6140, South Africa. E-mail: t.nyokong@ru.ac.za
bDmitry Mendeleev University of Chemical Technology of Russia, Miusskaya sq., 9, 125047, Moscow, Russia
cA. N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Leninskii pr., 31, bldg. 4, Moscow, 119071, Russia. E-mail: Martynov.Alexandre@gmail.com
dKurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Leninskii pr., 31, Moscow, 119991, Russia. E-mail: yulia@igic.ras.ru
First published on 31st October 2017
Abstract
Two alternative synthetic approaches affording a low-symmetry A3B-type phthalocyanine 1 bearing two [2′-(2′′-mercaptoethoxy)ethoxy] anchoring substituents were developed. Due to the presence of thiol groups, this phthalocyanine could be conjugated with TOPO-capped (TOPO - trioctylphosphine)-capped CdSe quantum dots (CdSe-QDs) or oleylamine capped silver nanoparticles (Ag-NPs). The nonlinear optical behaviour of starting phthalocyanine, quantum dots, nanoparticles and their conjugates was studied by using an open aperture Z-scan technique, revealing that the grafting of 1 onto the nanomaterials resulted in a significant enhancement of the optical limiting of 1-Ag and 1-CdSe in comparison with the individual components. The conjugate 1-CdSe, being the first example of Pc-based thiol conjugated with quantum dots, revealed superior limiting characteristics with a limiting threshold below 0.18 J cm−2.
Introduction
Optical limiting (OL) materials are important in protecting light sensitive materials from intense laser beams.1–6 The materials for OL are required to have some desirable nonlinear optical (NLO) properties which include large nonlinear absorption coefficients and fast response times to provide efficient optical limiting.Among the organic molecules that have been exploited for OL application, phthalocyanines (Pcs) have proven to be excellent NLO materials.1,7–10 This is due to their excellent photophysical properties and optical nonlinearities provided by the extended π-electron conjugated system, as well as high thermal and chemical stability, wide possibilities of synthesis and structural modification.11 The tuning of the NLO properties of Pcs can be achieved by varying the nature of the central metal in the cavity of the Pc ring, by altering the ring substituents or by lowering the molecular symmetry.12–15 For example, phthalocyanines containing heavy central metals such as In, Ga or Zn show improved NLO behaviour due to increased intersystem crossing as a result of the heavy atom effect.8,16,17 On the other hand, metal free phthalocyanines are also known to possess good NLO behaviour as a result of the lack of symmetry.18
Nanoparticles (NPs) and quantum dots (QDs) are also promising optical materials due to their quantum confinement.19–24 Conjugation of Pcs with nanomaterials improves the NLO behaviour of the conjugates in comparison with the parent species.25–27 Such conjugation typically requires the introduction of certain anchoring groups into the Pcs, and several examples of Pcs conjugated with QDs via amide or ester bonds have been previously reported, however, to the best of our knowledge the conjugates of QDs with thiolated Pcs have not been described. At the same time, thiolation of various organic substrates is a powerful tool for their further conjugation with nanoparticles, metal surfaces, etc.28,29 Thiolated Pcs have been shown to be valuable components of such conjugates, acting as sensors,30 photosensitizers,31 hole injectors,32etc.
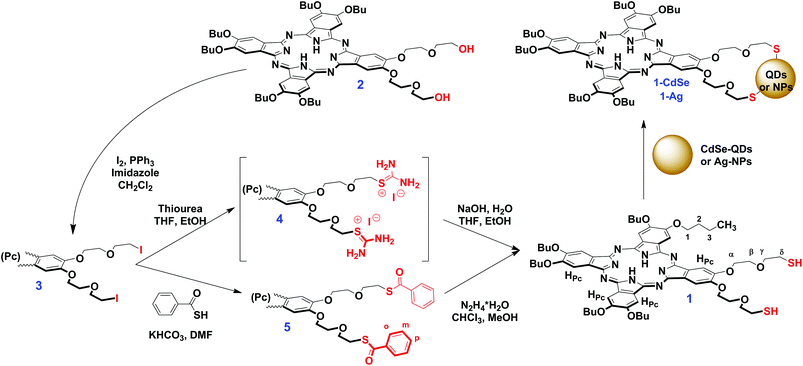
In the present study we report the synthesis of the novel low-symmetry phthalocyanine 1, starting from the recently published A3B-type ligand 2,18 bearing two diethyleneglycol chains with terminal OH-groups (Scheme 1). The asymmetry of ligand 2 and its complexes was shown to improve its NLO behaviour in comparison with symmetrical phthalocyanines,18,33 therefore we aimed to study the OL properties of the thiolated derivative 1 and to investigate the influence of its conjugation with various nanomaterials. This was studied herein on the examples the nanoconjugates 1-CdSe and 1-Ag which were formed by the interaction of 1 with trioctylphosphine oxide (TOPO) capped CdSe quantum dots (CdSe-QDs) and oleylamine capped silver nanoparticles (Ag-NPs). The nonlinear optical behaviour of the starting materials and conjugates was studied by using the open aperture Z-scan technique.
Results and discussion
Synthesis and characterization of phthalocyanines
Two major approaches of Pc thiolation were previously reported, namely the reaction of Pcs bearing terminal leaving groups with thiourea and the subsequent hydrolysis of the intermediate thiouronium salt32,34,35 or the direct functionalization of Pcs with thiol-containing fragments.31Using the first approach, we started the synthesis of the target thiolated phthalocyanine 1 from the previously reported A3B-type ligand 2, which was shown to exhibit prominent NLO properties due to the low molecular symmetry. To introduce the leaving groups, we treated the parent Pc 2 with iodine, triphenylphosphine and imidazole in DCM (Scheme 1). It afforded a diiodide derivative 3, which was isolated in 84% yield after column chromatography. The reaction of 3 with thiourea in the THF/EtOH mixture afforded the corresponding thiouronium salt 4 which was hydrolyzed without isolation with aqueous NaOH solution. The isolation of the dithiol 1 by column chromatography afforded the target compound in a moderate yield (39%). However, the harsh conditions of this reaction might preclude the application of this approach in the case of more sophisticated Pcs, like sandwich complexes, which are less tolerant to hydrolysis in strongly polar media.36–38
With the aim to improve the abovementioned drawbacks, we have proposed another approach, which implied the substitution of iodine atoms with thiobenzoyl fragments and the subsequent mild cleavage of benzoyl protective groups (Scheme 1).39 The treatment of 3 with thiobenzoic acid in the presence of excess of KHCO3 in DMF afforded thiobenzoylated Pc 5 which was isolated in 89% yield. The previously reported procedure of the deprotection of benzoyl groups with hydrazine hydrochloride and sodium acetate in DMF39 failed to furnish the target dithiol 1, however, Pc 5 could be smoothly and quantitatively deprotected by using hydrazine hydrate in the CHCl3/MeOH mixture.
Compounds 1, 3 and 5 were characterized by MALDI TOF MS, 1H-NMR, UV-Vis and FT-IR spectra and elemental analysis, which were in full agreement with the proposed structures (Fig. S1–S9†). 1H-NMR spectroscopy evidenced the non-equivalence of aromatic protons of the phthalocyanine macrocycle in low-symmetry ligands 1, 3 and 5 (Fig. 1, S1 and S5†), which arises from slightly different electronic effects of the butoxy group and the diethyleneglycol chain.18,33 In turn, it results in dissymmetry in the distribution of the electronic density over the ligand, which provides starting Pc 2 and its complexes with the enhanced NLO properties.18,33
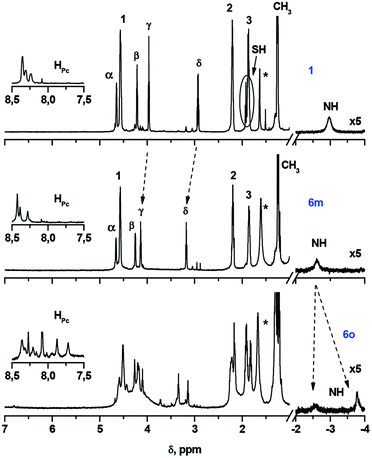 | ||
| Fig. 1 1H-NMR spectra of phthalocyanines 1, 6m and 6o in CDCl3. The numbering of protons is given in Scheme 1. The insets show the resonance signals of aromatic protons. Asterisk marks show the residual water signal. | ||
Although the presence of the SH-group in 1 was confirmed by FT-IR (a weak band at 2556 cm−1) and NMR spectra (triplet signal at 1.92 ppm and J = 8.2 Hz), in the MALDI-TOF mass-spectrum we observed the set of molecular ions [(M − 2H)n]+ with n = 1–3, which could correspond to the oxidative dimerization of SH-groups under the MS experimental conditions (Fig. S9†).
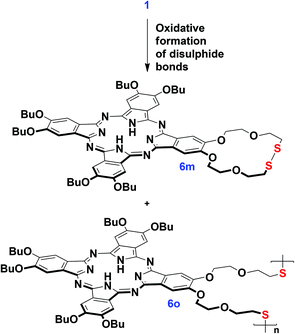
Moreover, the oxidation of 1 with the formation of disulphide derivatives with S–S bonds proceeded slowly upon the aerobic storage of 1 (Scheme 2). This feature is well-known in thiol chemistry and in our case the formation of disulphides can be facilitated due to the generation of singlet oxygen promoted by the phthalocyanine. Dithiol 1 can undergo the intra-/intermolecular oxidative coupling of SH-groups with the formation of dithiacrown-ether macrocycles.40 In contrast, the benzoylated ligand 5 is air stable, which makes the proposed pathway to the synthesis of thiolated Pcs more convenient in comparison with the methodology involving thiourea.41
We managed to separate the mixture of oxidation products and isolate the essentially pure monomeric phthalocyanine 6m and the mixture of oligomeric species 6o by size-exclusion chromatography on BioBeads S-X1. According to MALDI TOF MS, the fraction 6o contained the species from a dimer to a tetramer. 1H-NMR characterization of the isolated fractions indeed evidenced the disappearance of SH-group resonance in both fractions as well as the appearance of the complicated set of resonance signals in the spectrum of 6m and the splitting of the NH proton signal (Fig. 1). The broad Q-band, which was observed in the UV-Vis spectrum of 6o, is in agreement with the proposed oligomeric structure,42 while the UV-Vis spectrum of 6m was typical of monomeric phthalocyanines (Fig. S10†).
Nevertheless, the formation of disulphides is not an obstacle for further binding to nanomaterials, since they undergo easy reductive cleavage with the formation of metal–sulphur bonds.28–30
Conjugation of phthalocyanine 1 with quantum dots and nanoparticles
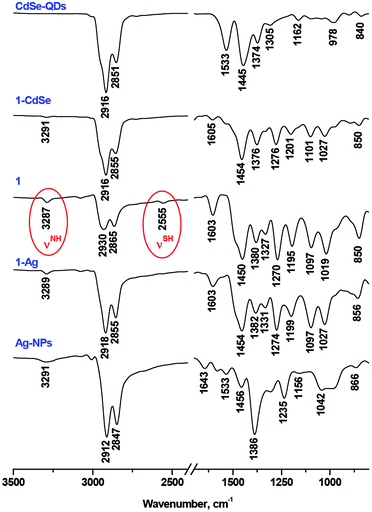
The reaction of 1 with CdSe-QDs and Ag-NPs proceeded in DCM solution via the substitution of TOPO and oleylamine ligands with thiols. This process was followed by the disappearance of the SH vibration band and the bands of capping ligands in the FT-IR spectra of 1-CdSe and 1-Ag conjugates, which suggested the formation of metal–sulphur bonds (Fig. 2).The UV-Vis spectrum of 1 alone consisted of well-resolved split Q-bands at 699 nm and 662 nm, which is typical of a monomeric metal-free Pc, but in the UV-Vis spectra of 1-CdSe and 1-Ag, the Pc Q-band regions were dominated by a broad band below 640 nm (Fig. 3). This band might be assigned both to the interaction between the electronic systems of Pc, QDs or NPs, as well as the aggregation of Pc molecules, absorbed on the surface of nanomaterials. The observed enhancement in the UV–Vis absorption spectra of the conjugates (1-CdSe and 1-Ag) around 350 nm to 650 nm compared to compound 1 alone could also be attributed to the presence of CdSe-QDs and Ag-NPs in the respective conjugates which absorb around 350 nm to 650 nm. This is evidenced by using the overlay of UV–Vis absorption for CdSe-QDs, 1 and their conjugates and further corroborated by XRD and TEM (Fig. 4 and 5). The presence of all expected elements was confirmed by EDX spectra (Fig. S11†).
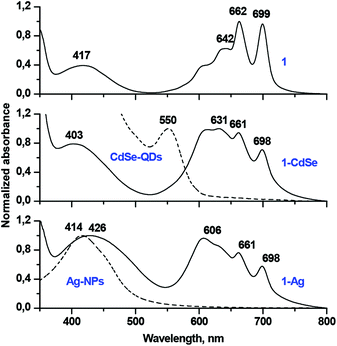 | ||
| Fig. 3 Normalized UV-Vis spectra of phthalocyanine 1, TOPO capped CdSe quantum dots, oleylamine capped silver nanoparticles alone, conjugates 1-CdSe and 1-Ag in DCM. | ||
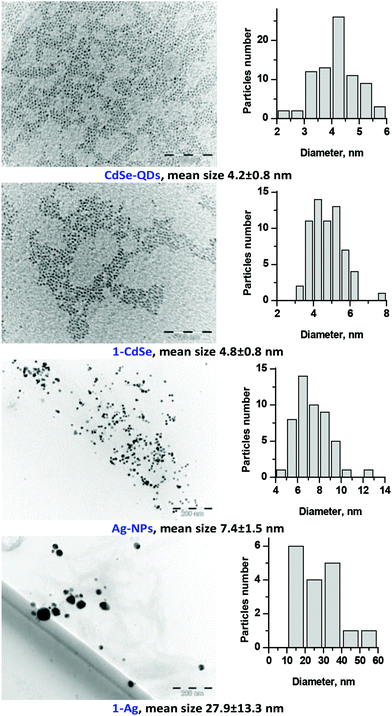 | ||
| Fig. 5 TEM images and the corresponding size distributions of TOPO-CdSe quantum dots, oleylamine-capped Ag nanoparticles and conjugates 1-CdSe and 1-Ag. | ||
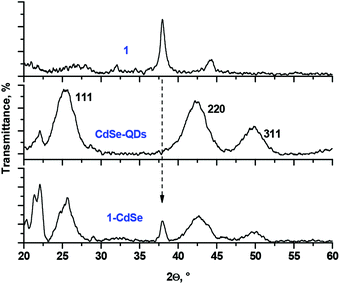
The XRD pattern of the conjugates resembled the patterns of both 1 and QDs or NPs (Fig. 4 showing the example of 1-CdSe). The XRD diffractogram of CdSe-QDs alone confirmed the presence of three prominent diffraction patterns with 2θ values (2θ = 25.29°, 42.29° and 49.86°) corresponding to the zinc blend crystal and cubic structure at planes 111, 220 and 311, respectively. The size of the CdSe-QDs (3.8 nm) was calculated based on the Debye–Scherrer equation.43 The nanoconjugate 1-CdSe also showed a similar diffraction pattern to the CdSe-QDs, but with additional sharp peaks which could imply an increase in crystallinity upon linking to Pcs. Ligand 1 alone showed crystalline peaks and this is expected for Pcs as reported in the literature.44
TEM measurements were used to characterize both starting QDs and NPs as well as the resulting conjugates (Fig. 5). The size of particles, found by TEM is close to those found by XRD. In the case of 1-Ag the aggregation of nanoparticles upon conjugation was observed which could alter the optical properties of the conjugates.45,46
Measurements of nonlinear optical properties
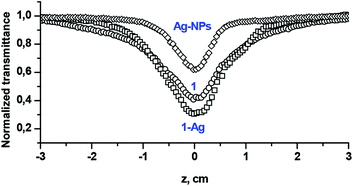
Fig. 6 shows the examples of the open aperture Z-scan profile spectra of Ag-NPs and Pc 1 alone as well as the 1-Ag conjugate, recorded at 532 nm with a 10 ns laser pulse in DCM. From the spectra, we can conclude that compound 1 and its nanoconjugate exhibit reverse saturable absorption (RSA) which is typical of phthalocyanines.4,12 The normalized transmittance of 1-Ag exhibited a deeper valley compared to complex 1 alone and Ag-NPs alone, suggesting a better OL performance of the conjugate. Similar results were obtained for CdSe-QDs and the 1-CdSe conjugate.The imaginary third-order nonlinear susceptibility Im[χ(3)] was calculated from the two photon absorption coefficient βeff; the values are listed in Table 1. Im[χ(3)] is related to the hyperpolarizability γ which measures the interaction of the incident photon with the permanent dipole moments of the Pcs. Im[χ(3)] measures the response time of a NLO material, following the perturbation initiated by the intense laser pulses. An efficient optical limiting material must have high γ and/or Im[χ(3)] as well as βeff. It was shown that the βeff and Im[χ(3)] are improved for 1 in the presence of QDs or NPs. The βeff and Im[χ(3)] values of the conjugates (1-CdSe and 1-Ag) were superior to those of the NPs or compound 1 alone, as shown in Table 1. This trend further lay credence to the synergistic activity that can be achieved when nanomaterials are linked to phthalocyanines.
| Compound | β eff (m W−1) | Im[χ(3)] (esu) | I lim (J cm−2) |
|---|---|---|---|
| a 50% attenuation of the transmitted fluence is not achieved. | |||
| 1 | 3.61 × 10−8 | 3.09 × 10−11 | 1.35 |
| 1 -CdSe | 7.30 × 10−8 | 6.26 × 10−11 | 0.18 |
| 1 -Ag | 5.76 × 10−8 | 4.94 × 10−11 | 0.43 |
| CdSe-QDs | 3.00 × 10−9 | 2.57 × 10−12 | |
| Ag-NPs | 1.53 × 10−8 | 1.31 × 10−11 | |
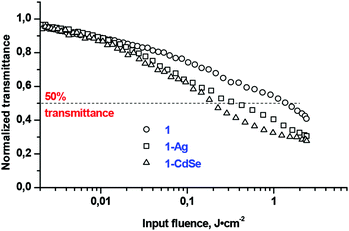
The investigated OL material displayed a decreasing transmittance as a function of the incident fluence (Fig. 7). At a low incident fluence, the material has a linear transmittance; while at some critical fluence or threshold, the transmittance changes abruptly, and leads to the clamping of the output fluence at a constant value that would presumably be less than the amount required to damage the optical element.5 This critical point is referred to as the threshold limit intensity or fluence (Ilim).5 The lower the Ilim values the better is the material as an optical limiter. The Ilim value decreased (Table 1) for the conjugates as compared to complex 1 alone, particularly indicating a superior optical limiting behaviour for the 1-CdSe composite material. 50% attenuation of the transmitted fluence is not achieved for the nanomaterials which show their deficiency when used alone since their conjugates with Pcs exhibited an improved limiting threshold pivotal for optical nonlinearity.
The improvement of OL in conjugates can be attributed to several factors. In the case of QDs, they absorb at 532 nm where the NLO behaviour was studied, therefore QDs can contribute to the NLO behaviour of Pcs due to the free-carrier absorption (FCA) mechanism, which is usually produced when excitation takes place at the wavelengths where there is linear absorption.26 Second, QDs and NPs may improve the NLO behaviour of Pcs due to the heavy atom effect which improves the triplet state population which directly affects the NLO behaviour. Finally, the aggregation of Pc chromophores in the vicinity of nanomaterials deduced from the UV-Vis spectra may also contribute to the enhancement of OL.45 Further studies might give more information about the synergistic effects which arise from electronic interaction between the phthalocyanine chromophore and quantum dots or nanoparticles.
Conclusions
We report for the first time, two synthetic approaches towards bis-[2′-(2′′-mercaptoethoxy)ethoxy]-substituted phthalocyanine 1, based on the thiolation of diiodo-derivative 3 – either by a conventional and relatively harsh reaction with thiourea and the subsequent hydrolysis of the intermediate thiouronium salt, or by a mild reaction of 3 with thiobenzoic acid and the quantitative deprotection of 5 with hydrazine. We envisage that the latter method can be used for the synthesis of more sophisticated thiolated Pcs which may not tolerate the conditions of the former approach.Dithiol 1 was conjugated with CdSe quantum dots and silver nanoparticles to study their effects on the nonlinear optical characteristics of the phthalocyanine chromophore within the nanoconjugates by the Z-scan technique. The two photon absorption mechanism of optical limiting was confirmed and the characteristic values of Im[χ(3)] and βeff were found. It is evidenced that the combination of 1 with QDs and NPs showed improved NLO behaviour compared to individual components. The mechanisms of this enhancement were discussed.
Experimental
Materials and methods
2,3-Bis[2′-(2′′-hydroxyethoxy)ethoxy]-9,10,16,17,23,24-hexa-n-butoxyphthalocyanine 2 was synthesized starting with 4,5-bis[2′-(2′′-hydroxyethoxy)ethoxy]benzene47 and 4,5-di-n-butoxyphthalonitrile according to the previously reported procedure.18 TOPO-capped CdSe-QDs and oleylamine-capped Ag-NPs were synthesized as reported in the literature.48,49Dichloromethane (DCM) was distilled over CaH2 under argon. Chloroform was stored over NaHCO3 and distilled over K2CO3 to remove acidic impurities. Tetrahydrofuran (THF) was distilled over Na/benzophenone under argon. Ultrapure water was obtained from a Milli-Q Water System (Millipore Corp., Bedford, MA, USA). Other chemicals (Merck, Aldrich), silica (Macherey Nagel) and BioBeads S-X1 (BioRad) were used as received from commercial suppliers.
The mass spectral data were acquired on a Bruker® Daltonics Ultraflex spectrometer with 2,4-dihydroxybenzoic acid as the matrix. A mass spectrometer operated in a positive ion mode using an m/z range of 500–5000 amu. NMR spectra were recorded with a Bruker Avance 600 spectrometer. NMR spectra were referenced against the residual solvent signal.50
Ground state electronic absorption was measured on Shimadzu UV-2550 and Thermo Evolution 201 spectrophotometers. FT-IR spectra were recorded on Bruker ALPHA FT-IR and Nexus Nicolet spectrometers with universal attenuated total reflectance (ATR) sampling accessories. X-ray powder diffraction patterns were recorded using Cu Kα radiation (1.5405°A, nickel filter) equipped with a Lynx Eye detector, on a Bruker® D8 Discover equipped with a proportional counter and the X-ray diffraction data were processed using the Eva (evaluation curve fitting) software. Elemental analyses were carried out on a EuroVector EA-3000 CHNS analyzer. The morphology of nanoparticles was assessed using a transmission electron microscope, ZEISS LIBRA® model 120, operated at 90 kV accelerating voltage. Z-Scan experiments were carried out as described in the literature using a frequency-doubled Nd:YAG laser (Quanta-Ray, 1.5 J/10 ns fwhm pulse duration) as the excitation source. Details have been provided previously.51
Elemental analysis, MALDI TOF MS, NMR and FT-IR measurements were performed at the Shared Facility Centres of the A.N. Frumkin Institute of Physical Chemistry and Electrochemistry, RAS, and the N.S. Kurnakov Institute of General and Inorganic Chemistry, RAS.
2,3-Bis[2′-(2′′-iodoethoxy)ethoxy]-9,10,16,17,23,24-hexa-n-butoxyphthalocyanine 3
Iodine (105 mg, 0.42 mmol) was weighed in a round bottom flask, 5 mL of DCM was added and the reaction mixture was stirred at an ambient temperature for 10 min, followed by the addition of triphenyphosphine (113 mg, 0.43 mmol). The reaction mixture was stirred for 30 min and then imidazole (56.5 mg, 0.75 mmol) was added into the reaction mixture which was allowed to stir for 10 min. The flask with the formed yellow suspension was protected from light with foil. Phthalocyanine 2 (100 mg, 87 μmol) was added and the mixture was stirred overnight. The progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion, aqueous Na2SO3 solution was added and the mixture was stirred for 10 min to quench the excess of the iodinating reagent. The organic layer was separated, washed with water and evaporated to give a dark-green residue. The iodinated derivative 3 was isolated by column chromatography on silica (elution with the mixture of CHCl3 + 1 vol% MeOH) as a dark-green solid (yield – 102 mg, 84%). MALDI TOF MS: calculated for C64H80I2N8O10 – 1374.5, found – 1374.4 [M]+. 1H NMR (600 MHz, CDCl3) δ 8.35, 8.34, 8.26 and 8.15 (4s, 2 × 2H, HPc), 4.61 (br m, 4H, α-CH2DEG), 4.58–4.53 (m, 12H, OCH2Bu), 4.24 (t, J = 4.8 Hz, 4H, β-CH2DEG), 4.13 and 3.51 (2t, J = 7.0 Hz, 2 × 4H, OCH2CH2I), 2.23–2.19 (m, 12H, 2-CH2Bu), 1.97–1.77 (m, 12H, 3-CH2Bu), 1.28–1.25 (m, 12H, CH2), −3.05 (br s, 2H, NH). UV-Vis (CHCl3) λmax (lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 702 (5.14), 665 (5.07), 603 (4.41), 426 (4.55), 348 (4.92), 296 (4.76). FT-IR, cm−1: 3290 (w, N–H stretching), 2955 (m), 2930 (m), 2869 (m), 1603 (m), 1536 (w), 1482 (s), 1444 (s), 1381 (s), 1328 (s), 1273 (s), 1197 (s), 1096 (s), 1020 (s), 932 (m), 852 (s), 796 (m), 739 (s), 691 (s), 613 (w), 576 (w). Elemental analysis (%): calc. for C64H80I2N8O10 (3): C, 55.90; H, 5.86; N, 8.15. Found: C, 55.47; H, 5.91; N, 8.33.
ε): 702 (5.14), 665 (5.07), 603 (4.41), 426 (4.55), 348 (4.92), 296 (4.76). FT-IR, cm−1: 3290 (w, N–H stretching), 2955 (m), 2930 (m), 2869 (m), 1603 (m), 1536 (w), 1482 (s), 1444 (s), 1381 (s), 1328 (s), 1273 (s), 1197 (s), 1096 (s), 1020 (s), 932 (m), 852 (s), 796 (m), 739 (s), 691 (s), 613 (w), 576 (w). Elemental analysis (%): calc. for C64H80I2N8O10 (3): C, 55.90; H, 5.86; N, 8.15. Found: C, 55.47; H, 5.91; N, 8.33.
2,3-Bis[2′-(2′′-benzoylthioethoxy)ethoxy]-9,10,16,17,23,24-hexa-n-butoxyphthalocyanine 5
Diiodide 3 (21 mg, 15 μmol) was suspended in 3 ml DMF, thiobenzoic acid (17 mg, 0.123 mmol) and KHCO3 (18 mg, 0.18 mmol) were added. The mixture was flushed with argon and heated to 45 °C overnight. Upon the completion of the reaction, which is confirmed by MALDI-TOF mass-spectrometry, the reaction mixture was diluted with water; the obtained green precipitate was filtered and washed off the filter with chloroform. The thiobenzoylated derivative 5 was isolated by column chromatography on silica (elution with the mixture of CHCl3 + 1 vol% MeOH) and size-exclusion chromatography in BioBeads S-X1 (elution with the mixture of CHCl3 + 2.5 vol% MeOH) as a dark-green solid (yield – 19 mg, 89%). MALDI TOF MS: calculated for C78H90N8O12S2 – 1394.6, found – 1394.8 [M]+. 1H NMR (600 MHz, CDCl3) δ, ppm: 8.25–8.20 (m, 8H, HPc), 8.00 (d, J = 7.7 Hz, 4H, o-CH), 7.46 (t, J = 7.3 Hz, 2H, p-CH), 7.36 (t, J = 7.7 Hz, 4H, m-CH), 4.64 (br s, 4H, α-CH2DEG), 4.54–4.51 (m, 12H, OCH2Bu), 4.25 (t, J = 4.7 Hz, 4H, β-CH2DEG), 4.06 and 3.52 (2t, J = 6.5 Hz, 2 × 4H, OCH2OCH2S), 2.22–2.17 (m, 12H, 2-CH2Bu), 1.89–1.84 (m, 12H, 3-CH2Bu), 1.27–1.23 (m, 18H, CH2), −3.29 (s, 2H, NH). UV-Vis (CHCl3) λmax (lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 702 (5.22), 665 (5.14), 603 (4.51), 426 (4.62), 349 (4.99), 295 (4.82). FT-IR, cm−1: 3290 (w, N–H stretching), 2955 (m), 2931 (m), 2870 (m), 1663 (m, C
ε): 702 (5.22), 665 (5.14), 603 (4.51), 426 (4.62), 349 (4.99), 295 (4.82). FT-IR, cm−1: 3290 (w, N–H stretching), 2955 (m), 2931 (m), 2870 (m), 1663 (m, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1601 (m), 1534 (w), 1487 (s), 1445 (s), 1382 (s), 1326 (s), 1275 (s), 1200 (s), 1094 (s), 1026 (s), 909 (s), 851 (s), 796 (m), 740 (s), 689 (s), 648 (m), 616 (w), 575 (w). Elemental analysis (%): calc. for C78H94N8O14S2 (5·2H2O): C, 65.43; H, 6.62; N, 7.83; S, 4.48. Found: C, 65.74; H, 6.16; N, 8.18; S, 4.44.
O stretching), 1601 (m), 1534 (w), 1487 (s), 1445 (s), 1382 (s), 1326 (s), 1275 (s), 1200 (s), 1094 (s), 1026 (s), 909 (s), 851 (s), 796 (m), 740 (s), 689 (s), 648 (m), 616 (w), 575 (w). Elemental analysis (%): calc. for C78H94N8O14S2 (5·2H2O): C, 65.43; H, 6.62; N, 7.83; S, 4.48. Found: C, 65.74; H, 6.16; N, 8.18; S, 4.44.
2,3-Bis[2′-(2′′-mercaptoethoxy)ethoxy]-9,10,16,17,23,24-hexa-n-butoxyphthalocyanine 1
Method 1. Diiodide 3 (70 mg, 51 μmol) and thiourea (40 mg, 0.526 mmol) were dissolved in the mixture of 10 ml THF and 3 ml EtOH, the mixture was degassed and refluxed at 80 °C under argon for 5 h to form the thiouronium salt 4. This salt without isolation was hydrolyzed by the addition of 2 ml of degassed 20% aq. NaOH solution and subsequent refluxing for 2 h. The mixture was neutralized with 0.63 ml of glacial acetic acid. Then it was transferred into the separating funnel, containing 25 ml of H2O and 10 ml of CHCl3. The organic layer was separated, washed with water and evaporated. The target dithiol 1 was isolated by column chromatography on silica (elution with the mixture of CHCl3 + 0–3 vol% MeOH) as a dark-green solid (yield – 24 mg, 39%).Method 2. Thiobenzoylated derivative 5 (12 mg, 8 μmol) was dissolved in the mixture of 2.5 ml CHCl3 and 1.25 ml MeOH, 0.1 ml of hydrazine hydrate was added and the mixture was heated to 40 °C under an argon atmosphere overnight. Upon completion of the reaction, confirmed by MALDI-TOF mass-spectrometry, the reaction mixture was washed with water in a separating funnel and the organic layer was evaporated to dryness, yielding target dithiol 1 with quantitatively cleaved protective groups.
MALDI TOF MS: calculated for C64H82N8O10S2 – 1186.6, found – 1184.5 [M − 2H]+. 1H NMR (600 MHz, CDCl3) δ 8.35, 8.30 and 8.24 (3s, 4H+2×2H, HPc), 4.65 (br s, 4H, α-CH2DEG), 4.59–4.54 (m, 12H, OCH2Bu), 4.21 (t, J = 4.7 Hz, 4H, β-CH2DEG), 3.97 (t, J = 6.5 Hz, 4H, γ-CH2DEG), 2.93 (dd, J = 14.4, 6.5 Hz, 4H, δ-CH2DEG), 2.20 (m, 12H, 2-CH2Bu), 1.92 (t, J = 8.2 Hz, 2H, SH), 1.89–1.85 (m, 12H, 3-CH2Bu), 1.28–1.23 (m, 18H, CH3), −2.98 (s, 2H, NH). UV-Vis (CH2Cl2) λmax (lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 700 (4.51), 663 (4.57), 643 (4.46), 419 (4.14), 347 (4.56). FT-IR, cm−1: 3287 (w, N–H stretching), 2930, 2865 (s), 2556 (SH stretching), 1603 (m), 1450 (s), 1380 (m), 1327 (m), 1270 (s), 1195 (m), 1097 (m), 1019 (m), 850 (m). Elemental analysis (%): calc. for C64H82N8O11S2 (1·H2O): C, 63.87; H, 6.87; N, 9.31; S, 5.33. Found: C, 63.65; H, 6.74; N, 9.33; S, 5.24.
ε): 700 (4.51), 663 (4.57), 643 (4.46), 419 (4.14), 347 (4.56). FT-IR, cm−1: 3287 (w, N–H stretching), 2930, 2865 (s), 2556 (SH stretching), 1603 (m), 1450 (s), 1380 (m), 1327 (m), 1270 (s), 1195 (m), 1097 (m), 1019 (m), 850 (m). Elemental analysis (%): calc. for C64H82N8O11S2 (1·H2O): C, 63.87; H, 6.87; N, 9.31; S, 5.33. Found: C, 63.65; H, 6.74; N, 9.33; S, 5.24.
Functionalization of CdSe-QDs and Ag-NPs with dithiol 1
TOPO capped CdSe-QDs (25 mg) or Ag-NPs (5 mg) were weighed in a flask, followed by the addition of 2 mL solution of 6 mg complex 1 in dichloromethane. It was stirred for 18 h. After this, the reaction mixture was evaporated to dryness. The obtained conjugate was characterized by UV-Vis spectroscopy, FTIR spectroscopy, XRD and TEM (see Results and discussion for more details).Z-Scan measurements
The nonlinear optical activities of phthalocyanine 1 and its nanoconjugates 1-CdSe and 1-Ag were tested using the open aperture Z-scan technique using eqn (1).5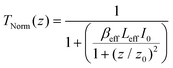 | (1) |
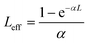 | (2) |
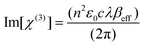 | (3) |
In eqn (3), c and n, respectively, are the speeds of light in vacuum and the linear refractive index, respectively, ε0 is the permittivity of free space and λ is the wavelength of the laser light.
At a molecular level, there is a direct correlation of Im[χ(3)] with the hyperpolarizability γ (which provides the nonlinear absorption per mole of the sample) via the relationship shown in eqn (4):8,52
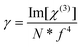 | (4) |
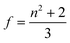 | (5) |
By definition, the limiting threshold (Ilim/J cm−2) is the input fluence at which the output fluence is 50% of the linear transmission.53,54 The results were found to fit into two photon absorption equation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Department of Science and Technology (DST), the National Research Foundation (NRF), the South Africa through DST/NRF South African Research Chairs Initiative for Professor of Medicinal Chemistry and Nanotechnology (UID = 62620) as well as Rhodes University. This work was also supported by the Programs of Russian Academy of Sciences.Notes and references
- D. Dini, M. J. F. Calvete and M. Hanack, Chem. Rev., 2016, 116, 13043–13233 CrossRef CAS PubMed.
- J. E. Riggs and Y.-P. Sun, J. Phys. Chem. A, 1999, 103, 485–495 CrossRef CAS.
- Y.-P. Sun and J. E. Riggs, Int. Rev. Phys. Chem., 1999, 18, 43–90 CrossRef CAS.
- L. De Boni, D. S. Corrêa and C. R. Mendonça, in Advances in Lasers and Electro Optics, ed. N. Costa and A. Cartaxo, InTech, 2010, pp. 33–58 Search PubMed.
- R. L. Sutherland, Handbook of Nonlinear Optics, Revised and Expanded, Marcel Dekker, Inc., New York, Basel, 2nd edn, 2003 Search PubMed.
- J. L. Bredas, C. Adant, P. Tackx, A. Persoons and B. M. Pierce, Chem. Rev., 1994, 94, 243–278 CrossRef CAS.
- D. Dini, M. Barthel and M. Hanack, Eur. J. Org. Chem., 2001, 2001, 3759–3769 CrossRef.
- Y. Chen, M. Hanack, Y. Araki and O. Ito, Chem. Soc. Rev., 2005, 34, 517–529 RSC.
- J. W. Perry, D. Alvarez, I. Choong, K. Mansour, S. R. Marder and K. J. Perry, Opt. Lett., 1994, 19, 625 CrossRef CAS PubMed.
- A. G. Martynov, Y. G. Gorbunova and A. Y. Tsivadze, Russ. J. Inorg. Chem., 2014, 59, 1635–1664 CrossRef CAS.
- V. N. Nemykin, S. V. Dudkin, F. Dumoulin, C. Hirel, A. G. Gürek and V. Ahsen, ARKIVOC, 2014, 2014, 142 CrossRef.
- G. de la Torre, P. Vázquez, F. Agulló-López and T. Torres, J. Mater. Chem., 1998, 8, 1671–1683 RSC.
- G. de la Torre, P. Vázquez, F. Agulló-López and T. Torres, Chem. Rev., 2004, 104, 3723–3750 CrossRef CAS PubMed.
- E. M. García-Frutos, S. M. O'Flaherty, E. M. Maya, G. de la Torre, W. Blau, P. Vázquez and T. Torres, J. Mater. Chem., 2003, 13, 749–753 RSC.
- S. J. Mathews, S. Chaitanya Kumar, L. Giribabu and S. Venugopal Rao, Opt. Commun., 2007, 280, 206–212 CrossRef CAS.
- V. Chauke, M. Durmuş and T. Nyokong, J. Photochem. Photobiol., A, 2007, 192, 179–187 CrossRef CAS.
- J. S. Shirk, R. G. S. Pong, S. R. Flom, H. Heckmann and M. Hanack, J. Phys. Chem. A, 2000, 104, 1438–1449 CrossRef CAS.
- J. Britton, A. G. Martynov, D. O. Oluwole, Y. G. Gorbunova, A. Y. Tsivadze and T. Nyokong, J. Porphyrins Phthalocyanines, 2016, 20, 1296–1305 CrossRef CAS.
- W. Jia, E. P. Douglas, F. Guo and W. Sun, Appl. Phys. Lett., 2004, 85, 6326–6328 CrossRef CAS.
- S. Qu, C. Du, Y. Song, Y. Wang, Y. Gao, S. Liu, Y. Li and D. Zhu, Chem. Phys. Lett., 2002, 356, 403–408 CrossRef CAS.
- N. Venkatram, D. N. Rao and M. a. Akundi, Opt. Express, 2005, 13, 867–872 CrossRef CAS PubMed.
- R. B. Martin, M. J. Meziani, P. Pathak, J. E. Riggs, D. E. Cook, S. Perera and Y. P. Sun, Opt. Mater., 2007, 29, 788–793 CrossRef CAS.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435–446 CrossRef CAS PubMed.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- J. Britton, C. Litwinski, M. Durmuş, V. Chauke and T. Nyokong, J. Porphyrins Phthalocyanines, 2011, 15, 1239–1249 CrossRef CAS.
- K. Sanusi, S. Khene and T. Nyokong, Opt. Mater., 2014, 37, 572–582 CrossRef CAS.
- J. Britton, M. Durmuş, V. Chauke and T. Nyokong, J. Mol. Struct., 2013, 1054–1055, 209–214 CrossRef CAS.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed.
- J. C. Love, L. a. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1170 CrossRef CAS PubMed.
- T. R. E. Simpson, D. A. Russell, I. Chambrier, M. J. Cook, A. B. Horn and S. C. Thorpe, Sens. Actuators, B, 1995, 29, 353–357 CrossRef CAS.
- N. Nombona, E. Antunes, C. Litwinski and T. Nyokong, Dalton Trans., 2011, 40, 11876 RSC.
- X. Huang, Y. Liu, S. Wang, S. Zhou and D. Zhu, Chem. – Eur. J., 2002, 8, 4179–4184 CrossRef CAS.
- D. O. Oluwole, A. V. Yagodin, N. C. Mkhize, K. E. Sekhosana, A. G. Martynov, Y. G. Gorbunova, A. Y. Tsivadze and T. Nyokong, Chem. – Eur. J., 2017, 23, 2820–2830 CrossRef CAS PubMed.
- T. Kimura, T. Suzuki, Y. Takaguchi, A. Yomogita, T. Wakahara and T. Akasaka, Eur. J. Org. Chem., 2006, 1262–1270 CrossRef CAS.
- I. Chambrier, M. J. Cook and D. A. Russell, Synthesis, 1995, 1283–1286 CrossRef CAS.
- K. P. Birin, Y. G. Gorbunova and A. Y. Tsivadze, Russ. J. Inorg. Chem., 2007, 52, 191–196 CrossRef.
- N. Sheng, Y. Zhang, H. Xu, M. Bao, X. Sun and J. Jiang, Eur. J. Inorg. Chem., 2007, 3268–3275 CrossRef CAS.
- A. G. Martynov, I. V. Nefedova, Y. G. Gorbunova and A. Y. Tsivadze, Mendeleev Commun., 2007, 17, 66–67 CrossRef CAS.
- R. L. A. David and J. A. Kornfield, Macromolecules, 2008, 41, 1151–1161 CrossRef CAS.
- S. Shinkai, K. Inuzuka, O. Miyazaki and O. Manabe, J. Org. Chem., 1984, 49, 3440–3442 CrossRef CAS.
- F. Goethals, D. Frank and F. Du Prez, Prog. Polym. Sci., 2017, 64, 76–113 CrossRef CAS.
- K. P. Birin, V. N. Chugunov, A. A. Krapivenko, Y. G. Gorbunova and A. Y. Tsivadze, Macroheterocycles, 2014, 7, 153–161 CrossRef CAS.
- R. Jenkins and R. Snyder, Introduction to X-Ray Powder Diffractometry, Wiley, 1996 Search PubMed.
- M. Ohmori, C. Nakano, A. Fujii, Y. Shimizu and M. Ozaki, J. Cryst. Growth, 2016, 0–1 Search PubMed.
- Y. G. Gorbunova, A. D. Grishina, A. G. Martynov, T. V. Krivenko, A. A. Isakova, V. V. Savel'ev, S. E. Nefedov, E. V. Abkhalimov, A. V. Vannikov and A. Y. Tsivadze, J. Mater. Chem. C, 2015, 3, 6692–6700 RSC.
- A. G. Martynov, I. V. Biryukova, Y. G. Gorbunova and A. Y. Tsivadze, Russ. J. Inorg. Chem., 2004, 49, 358–363 Search PubMed.
- A. G. Martynov, Y. G. Gorbunova, S. E. Nefedov, A. Y. Tsivadze and J.-P. Sauvage, Eur. J. Org. Chem., 2012, 2012, 6888–6894 CrossRef CAS.
- X. D. Luo, U. Farva, N. T. N. Truong, K. S. Son, P. S. Liu, C. Adachi and C. Park, J. Cryst. Growth, 2012, 339, 22–30 CrossRef CAS.
- D. O. Oluwole, E. Prinsloo and T. Nyokong, Polyhedron, 2016, 119, 434–444 CrossRef CAS.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, Organometallics, 2010, 29, 2176–2179 CrossRef CAS.
- K. Sanusi, E. Antunes and T. Nyokong, Dalton Trans., 2014, 43, 999–1010 RSC.
- D. Dini and M. Hanack, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guiard, Elsevier, 2003, pp. 1–36 Search PubMed.
- F. Li, Z. He, M. Li and P. Lu, Mater. Lett., 2013, 111, 81–84 CrossRef CAS.
- Y. Chen, L. Gao, M. Feng, L. Gu, N. He, J. Wang, Y. Araki, W. Blau and O. Ito, Mini-Rev. Org. Chem., 2009, 6, 55–65 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR, UV-Vis, FT-IR, MALDI TOF MS, EDX data for synthesized compounds and conjugates. See DOI: 10.1039/c7dt03867d |
| This journal is © The Royal Society of Chemistry 2017 |