Open Access Article
Open Access ArticleAddition of azomethine ylides to carbon-encapsulated iron nanoparticles†
Artur
Kasprzak
 *a,
Anna M.
Nowicka
b,
Jakub P.
Sek
b,
Maciej
Fronczak
*a,
Anna M.
Nowicka
b,
Jakub P.
Sek
b,
Maciej
Fronczak
 b,
Michał
Bystrzejewski
b,
Michał
Bystrzejewski
 b,
Mariola
Koszytkowska-Stawinska
a and
Magdalena
Poplawska
a
b,
Mariola
Koszytkowska-Stawinska
a and
Magdalena
Poplawska
a
aFaculty of Chemistry, Warsaw University of Technology, Noakowskiego Str. 3, 00-664 Warsaw, Poland. E-mail: akasprzak@ch.pw.edu.pl
bFaculty of Chemistry, University of Warsaw, Pasteura Str. 1, 02-093 Warsaw, Poland
First published on 29th November 2017
Abstract
The Prato reaction was applied for the covalent introduction of a variety of organic moieties onto carbon-encapsulated iron nanoparticles. The developed method is versatile and employs a broad range of commercially available reactants, including both aromatic and aliphatic aldehydes. The reported functionalization route provides high functionalization yields (ca. 12–21 wt%).
The cycloaddition reaction is one of the most commonly used synthetic routes to functionalize carbon nanostructures for various applications. Since the discovery of fullerenes in 1985 a number of their possible modifications have been developed, including the cycloaddition protocols.1–3 Over the years, it has been found that such a methodology can also be applied for the surface modification of carbon nanotubes4–6 and graphene.7–9 Graphene, in principal, has a flat structure and does not contain topological defects which introduce the local curvature and in consequence its reactivity is lower in comparison with fullerenes and carbon nanotubes. Such a phenomenon influences the reaction conditions for the functionalization of graphene, especially higher temperatures and longer reaction times are needed. Nevertheless, there are a number of cycloaddition reactions possible to conduct with the graphene layer. Many examples were recently summarized by Prato and co-workers.10
Carbon-encapsulated magnetic nanoparticles constitute as a family of carbon nanostructures. They comprise a magnetic core which is covered by curved graphene layers.11,12 The carbon shell protects the metallic core against environmental factors, e.g. oxidation and aggregation/agglomeration. Carbon-encapsulated magnetic nanoparticles possess a wide range of possible applications, including e.g. adsorption,13–15a sensors15b and nanomedicine.16–18 Several types of radical reactions with the exterior graphene layer of carbon-encapsulated magnetic nanoparticles have been established to date, including diazo13 and peroxide chemistry,19 as well as the direct amination protocol.20 These functionalization routes constitute as a direct link to the chemistry of fullerenes21,22 and carbon nanotubes.23,24 Importantly, the addition of nitrile oxides to carbon-encapsulated magnetic nanoparticles can also be conducted.25 If so, one can conclude that this magnetic graphene-related material can act as an active dipolarophile in the cycloaddition reaction like other carbon allotropes.
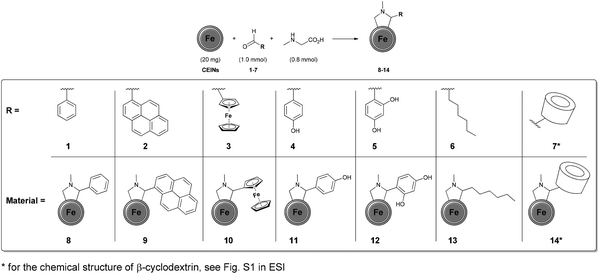
The Prato reaction is one of the most popular methods to functionalize carbon nanostructures. It is based on the 1,3-cycloaddition of azomethine ylides generated from the corresponding amino acids and aldehydes. The leading role of this reaction in the modification of carbon allotropes results from (i) effortless of the manual operation, (ii) employing commercially available reactants and (iii) providing high functionalization yields. The Prato reaction has been reported for fullerenes,27 carbon nanotubes,28 as well as for graphene.29 To the best of our knowledge the addition of azomethine ylides to the carbon-encapsulated magnetic nanoparticles has not been reported yet. In this paper, we report the syntheses of new derivatives of carbon-encapsulated iron nanoparticles (CEINs) using the Prato reaction. It is shown that various types of functional moieties can be efficiently introduced onto the surface of CEINs by employing the developed process.
The carbon arc discharge route was employed to obtain CEINs, which have a diameter between 10 and 100 nm.30 The magnetic core has a diameter of 10–30 nm, whilst the carbon shell is composed of ca. 5–20 graphene layers. The developed functionalization route (Fig. 1) involved refluxing CEINs (20 mg) with aldehydes (1.0 mmol) in the presence of sarcosine (0.8 mmol). The toluene or toluene/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system was used as the reaction medium. For experimental details see the ESI.† The mass gain was observed for each material. However, this feature only indicates the success of the reaction, because CEINs-based materials are highly hygroscopic. Fourier-transform infrared (FT-IR) spectroscopy and thermogravimetry (TGA) were performed to confirm the success of functionalization.
1) system was used as the reaction medium. For experimental details see the ESI.† The mass gain was observed for each material. However, this feature only indicates the success of the reaction, because CEINs-based materials are highly hygroscopic. Fourier-transform infrared (FT-IR) spectroscopy and thermogravimetry (TGA) were performed to confirm the success of functionalization.
 | ||
| Fig. 1 The developed method for the functionalization of CEINs. *For the chemical structure of β-cyclodextrin, see Fig. S1 in the ESI.† | ||
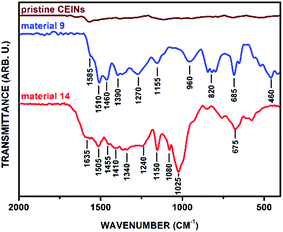
FT-IR spectroscopy provided qualitative information about the structure of the obtained materials. Firstly, the non-substituted aromatic aldehydes (1–2) were attached to the CEINs surface. The FT-IR spectrum of the representative material 9 is presented in Fig. 2 (for the spectrum of material 8 see Fig. S7 in the ESI†). The FT-IR spectrum of pristine CEINs is also shown. The weak absorption band at 1585 cm−1 is observed in the FT-IR spectrum of pristine CEINs only. In contrast, the spectra of the obtained materials consist of several strong absorption bands. As for example, the main features for material 9 are located at 1585, 1510, 1460, 1390, 1270, 1155, 960, 820, 685, and 460 cm−1. The characteristic absorption bands at 1585, 820, 685, and 640 cm−1 were assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and out-of-plane deformation vibrations. The adsorption bands at 1510, 1460, 1390, 1270, 1155, and 960 cm−1 were assigned to the vibrations of the N-methylpyrrolidine ring (C–N vibrations associated with the presence of the tertiary amine group as well as C–C vibrations). Importantly, no characteristic absorption band for the aldehyde group of the starting reactant (ca. 1730–1670 cm−1; see data in section S5.2 in the ESI†) was observed. This finding supports the conclusion that the organic moieties were covalently attached to CEINs. Obviously, the substituted N-methylpyrrolidine ring is formed on the surface of the functionalized CEINs.
C stretching and out-of-plane deformation vibrations. The adsorption bands at 1510, 1460, 1390, 1270, 1155, and 960 cm−1 were assigned to the vibrations of the N-methylpyrrolidine ring (C–N vibrations associated with the presence of the tertiary amine group as well as C–C vibrations). Importantly, no characteristic absorption band for the aldehyde group of the starting reactant (ca. 1730–1670 cm−1; see data in section S5.2 in the ESI†) was observed. This finding supports the conclusion that the organic moieties were covalently attached to CEINs. Obviously, the substituted N-methylpyrrolidine ring is formed on the surface of the functionalized CEINs.
 | ||
| Fig. 2 FT-IR spectra of pristine CEINs, material 9 and material 14. For legends, see Fig. 1. | ||
The success of such preliminary studies prompted us to broaden up the range of the aldehydes employed in the reported process. Ferrocenecarboxaldehyde (3) was chosen as the representative metallocene compound. The covalent attachment of the ferrocene (Fc) moiety was confirmed by FT-IR spectroscopy (material 10, Fig. S8 in the ESI†). The attachment of the aromatic moieties substituted with hydroxyl groups, namely 4-hydroxybenzaldehyde (4) and 2,4-dihydroxybenzaldehyde (5), was also achieved (FT-IR spectra of materials 11 and 12 are presented in Fig. S9 and S10 in the ESI,† respectively). The addition reactions with the hydroxyl-substituted aldehydes were conducted in the DMSO/toluene solvent system. Subsequently, heptanal (6) was applied as the representative aliphatic aldehyde. Once again, FT-IR spectroscopy was performed to confirm the success of the covalent attachment of the aliphatic moiety. The characteristic absorption bands for both the ligand and N-methylpyrrolidine ring were found in the spectrum of the material 13 (Fig. S11 in the ESI†). The addition of the azomethine ylide generated from the β-cyclodextrin aldehyde (βCD-CHO, 7)31 to the surface of CEINs was also succeeded. The characteristic strong absorption bands resulting from βCD (especially C–O–C vibrations arising from the glucose units, i.e. 1080 and 1025 cm−1) and the N-methylpyrrolidine ring were found in the FT-IR spectrum of the obtained carbon material (14, Fig. 2). No characteristic absorption band for the aldehyde group of βCD-CHO (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1720 cm−1, Fig. S6 in the ESI†) was observed.
O, 1720 cm−1, Fig. S6 in the ESI†) was observed.
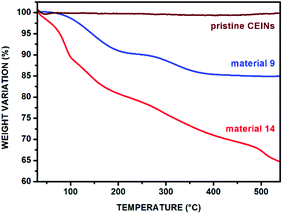
The content of the introduced organic moieties was calculated from the thermogravimetric (TGA) curves. The representative TGA curves (acquired in nitrogen) of materials 9 and 14 are presented in Fig. 3 (for other TGA curves see Fig. S13–S17 in the ESI†). The TGA curve for pristine CEINs is also shown. The first weight loss is observed between ca. 65 and 105 °C for both pristine CEINs and the corresponding materials. It is associated with the presence of moisture in the samples. The next weight loss up to 550 °C is observed only for the materials 8–14 and is directly attributed to the thermal decomposition of the introduced moieties. This temperature range is typical of the decomposition of covalently attached moieties to CEINs.20,26,32 The functionalization yields ranging from 12 wt% to 21 wt% are summarized in Table 1. The details of the calculation are presented in the ESI (section S6†). The highest content of the introduced moiety was found for material 10 bearing the Fc unit (21.2%) and material 14 bearing the βCD moiety (20.8%).
 | ||
| Fig. 3 TGA curve (in nitrogen) of pristine CEINs, materials 9 and 14. For legends, see Fig. 1. | ||
| Material no. | Content of introduced moiety [wt%] | Material no. | Content of introduced moiety [wt%] |
|---|---|---|---|
| 8 | 11.8 | 12 | 18.2 |
| 9 | 13.3 | 13 | 12.0 |
| 10 | 21.2 | 14 | 20.8 |
| 11 | 17.0 |
To exclude a possible non-covalent adsorption of organic moieties onto CEINs, the representative organic aldehydes 2, 3 and 5 (1.0 mmol) were refluxed with CEINs (20 mg) in the absence of sarcosine. As expected, the lack of sarcosine, the crucial reaction component for the formation of the intermediate ylide, prevented the covalent functionalization of CEINs. No weight gain was observed. Moreover, no characteristic absorption bands for the aldehydes in the FT-IR spectra were observed. Furthermore, no weight loss in the TGA curves of the obtained carbon materials was observed (data presented in section S7 in the ESI†). These findings revealed that the studied organic compounds were not permanently adsorbed onto the CEINs. Consequently, our thesis on the covalent modification of CEINs in the course of the developed functionalization route was supported.
Carbon nanomaterial 10 with the Fc unit was expected to show an interesting electroactivity. To explore its electrochemical properties and to determine the potential range of electroactivity, nanomaterial 10 was physically adsorbed on the glassy carbon electrode and subjected to the cyclic voltammetry experiments in the range of positive potentials. Both well-defined one anodic peak and one cathodic peak are observed in the cyclic voltammogram as presented in Fig. 4. These peaks confirm the successful conjugation of Fc residues to CEINs. The shift of the current signals on the potential scale in comparison with the unsubstituted ferrocene33 is a consequence of the interactions between the electron-donating ligand and the cyclopentadienyl ring. The calculated amount of Fc constituted ca. 18 wt% of the total weight of the carbon material 10 adsorbed at the electrode surface. This number is in very good agreement with the TGA data (ca. 21 wt%). It means that almost all Fc residues attached to the CEINs surface are capable of electron exchange with the electrode. The detailed electrochemical characteristic of the carbon material 10 is presented in section S8, ESI.†
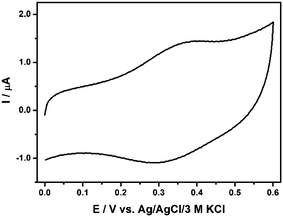 | ||
| Fig. 4 Cyclic voltammograms of material 10 adsorbed on the glassy carbon surface in 0.02 M PB buffer containing 0.15 M K2SO4, pH 7.40. Experimental conditions: T = 21 °C; scan rate 100 mV s−1; ∅ GC 3 mm. For legend, see Fig. 1. | ||
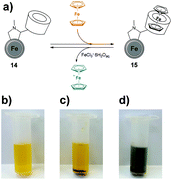
βCD is a well-known host molecule that participates in the formation of the supramolecular host–guest systems.34,35 Hence, the immobilization of β-cyclodextrin (βCD) onto CEINs (material 14) gives a possibility to bind hydrophobic chemical molecules to the functionalized carbon material. In particular, the interactions between βCD and Fc are of significant interest because of the high Fc@βCD association constant and the reversibility of the inclusion. When Fc is oxidized to an ferrocenium cation (Fc+) no complex with βCD is formed.36,37 We have examined the possibility of complexing the Fc inside the CEINs–βCD material and the subsequent release of Fc+ using the oxidizer. Importantly, after complexation of Fc inside βCD in material 14, Fc could be released from the complex (material 15) using the aqueous solution of iron(III) chloride (Fig. 5a, for experimental details see section S3.4 and discussion in section S5.3 in the ESI†). This process is visualized in Fig. 5b–d. When material 14 was sonicated in an aqueous solution of FeCl3·6H2O and then centrifuged, no color change of the supernatant was observed in comparison with FeCl3aq (Fig. 5b and c). In contrast, when material 15 was subjected to the same experiment, the color of the supernatant changed (Fig. 5d). This difference was associated with the oxidation of Fc to Fc+ followed by its release from the complex.
Conclusions
We have succeeded in the covalent functionalization of carbon-encapsulated iron nanoparticles (CEINs) via the addition of azomethine ylides. The developed method is a one-step process and employs commercially available reactants. Both aromatic and aliphatic aldehydes, as well as metallocene and sugar aldehydes were used in the developed process. The presented functionalization approach enables one to functionalize CEINs both with small organic ligands and unique supramolecules (cyclodextrin). The content of the introduced organic moiety was between 12 and 21%. For material 10 bearing the ferrocene unit, the characteristic electrochemical signals were highly consistent with the data for the ferrocene compound. This is a first example of the ferrocene–CEINs conjugate with well-defined electrochemical performance. Material 14 containing β-cyclodextrin units exhibited interesting complexation features, which have been proven by demonstrating the reversible host–guest interactions between β-cyclodextrin and ferrocene. In other words, the characteristic properties of the organic moieties were retained after covalent immobilization onto CEINs. The developed functionalization route opens up a way to modify magnetic carbon-related materials for a variety of applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported partially by the National Science Center (Poland) through the Grant PRELUDIUM No. 2016/21/N/ST5/00864 and the Warsaw University of Technology.Notes and references
- M. Prato, J. Mater. Chem., 1997, 7, 1097–1109 RSC.
- J.-F. Nierengarten, New J. Chem., 2004, 1177–1191 RSC.
- N. Zydziak, B. Yameen and C. Barner-Kowollik, Polym. Chem., 2013, 4, 4072 RSC.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS PubMed.
- P. Singh, S. Campidelli, S. Giordani, D. Bonifazi, A. Bianco and M. Prato, Chem. Soc. Rev., 2009, 38, 2214 RSC.
- M. Alvaro, P. Atienzar, P. De La Cruz, J. L. Delgado, V. Troiani, H. Garcia, F. Langa, A. Palkar and L. Echegoyen, J. Am. Chem. Soc., 2006, 128, 6626–6635 CrossRef CAS PubMed.
- A. Hirsch, J. M. Englert and F. Hauke, Acc. Chem. Res., 2013, 46, 87–96 CrossRef CAS PubMed.
- S. Sarkar, E. Bekyarova, S. Niyogi and R. C. Haddon, J. Am. Chem. Soc., 2011, 133, 3324–3327 CrossRef CAS PubMed.
- J. Li, M. Li, L. L. Zhou, S. Y. Lang, H. Y. Lu, D. Wang, C. F. Chen and L. J. Wan, J. Am. Chem. Soc., 2016, 138, 7448–7451 CrossRef CAS PubMed.
- M. Quintana, E. Vazquez and M. Prato, Acc. Chem. Res., 2013, 46, 138–148 CrossRef CAS PubMed.
- M. Bystrzejewski, A. Huczko and H. Lange, Sens. Actuators, B, 2005, 109, 81–85 CrossRef CAS.
- G. Wang, G. Wan and C. Hao, Mod. Phys. Lett. B, 2009, 23, 2149–2153 CrossRef CAS.
- R. N. Grass, E. K. Athanassiou and W. J. Stark, Angew. Chem., Int. Ed., 2007, 46, 4909–4912 CrossRef CAS PubMed.
- P. Strachowski and M. Bystrzejewski, Colloids Surf., A, 2015, 467, 113–123 CrossRef CAS.
- (a) R. Fuhrer, I. K. Herrmann, E. K. Athanassiou, R. N. Grass and W. J. Stark, Langmuir, 2011, 27, 1924–1929 CrossRef CAS PubMed; (b) E. Matysiak, M. Donten, A. Kowalczyk, M. Bystrzejewski, I. P. Grudzinski and A. M. Nowicka, Biosens. Bioelectron., 2015, 64, 554–559 CrossRef CAS PubMed.
- I. P. Grudzinski, M. Bystrzejewski, M. A. Cywinska, A. Kosmider, M. Poplawska, A. Cieszanowski and A. Ostrowska, J. Nanopart. Res., 2013, 16, 1835 CrossRef PubMed.
- G. Modugno, C. Ménard-Moyon, M. Prato and A. Bianco, Br. J. Pharmacol., 2015, 172, 975–991 CrossRef CAS PubMed.
- J. K. Park, J. Jung, P. Subramaniam, B. P. Shah, C. Kim, J. K. Lee, J. H. Cho, C. Lee and K. B. Lee, Small, 2011, 7, 1647–1652 CrossRef CAS PubMed.
- M. Poplawska, M. Bystrzejewski, I. P. Grudzinski, M. A. Cywinska, J. Ostapko and A. Cieszanowski, Carbon, 2014, 74, 180–194 CrossRef CAS.
- A. Kasprzak, M. Poplawska, M. Bystrzejewski and I. P. Grudzinski, J. Mater. Chem. B, 2016, 4, 5593–5607 RSC.
- G. P. Miller, C. R. Chim., 2006, 9, 952–959 CrossRef CAS.
- X. Ma and Y. Zhao, Chem. Rev., 2015, 115, 7794–7835 CrossRef CAS PubMed.
- E. V. Basiuk, M. Monroy-Peláez, I. Puente-Lee and V. A. Basiuk, Nano Lett., 2004, 4, 863–866 CrossRef CAS.
- Y. Ying, R. K. Saini, F. Liang, A. K. Sadana and W. E. Billups, Org. Lett., 2003, 1471–1473 CrossRef CAS PubMed.
- M. Poplawska, G. Z. Zukowska, S. Cudziło and M. Bystrzejewski, Carbon, 2010, 48, 1318–1320 CrossRef CAS.
- A. Kasprzak, M. Bystrzejewski, M. Koszytkowska-Stawinska and M. Poplawska, Green Chem., 2017, 19, 3510 RSC.
- M. Maggini, G. Scorrano and M. Prato, J. Am. Chem. Soc., 1993, 115, 9798–9799 CrossRef CAS.
- J. J. Mulvey, E. N. Feinberg, S. Alidori, M. R. McDevitt, D. A. Heller and D. A. Scheinberg, Int. J. Nanomed., 2014, 9, 4245–4255 CAS.
- M. Quintana, K. Spyrou, M. Grzelczak, W. R. Browne, P. Rudolf and M. Prato, ACS Nano, 2010, 4, 3527–3533 CrossRef CAS PubMed.
- M. Bystrzejewski, O. Łabedź, W. Kaszuwara, A. Huczko and H. Lange, Powder Technol., 2013, 246, 70–15 CrossRef.
- M. J. Cornwell, J. B. Huff and C. Bieniarz, Tetrahedron Lett., 1995, 36, 8371–8374 CrossRef CAS.
- A. Kasprzak, M. Poplawska, M. Bystrzejewski, O. Labedz and I. P. Grudzinski, RSC Adv., 2015, 5, 85556–85567 RSC.
- N. G. Tsierkezos, J. Solution Chem., 2007, 36, 289–302 CrossRef CAS.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1753 CrossRef CAS PubMed.
- G. Crini, Chem. Rev., 2014, 114, 10940–10975 CrossRef CAS PubMed.
- A. Harada and S. Takahashi, J. Chem. Soc., Chem. Commun., 1984, 645 RSC.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; TGA, FT-IR and NMR data; electrochemical measurements. See DOI: 10.1039/c7dt03689b |
| This journal is © The Royal Society of Chemistry 2018 |