Engineering transition metal phosphide nanomaterials as highly active electrocatalysts for water splitting
Abstract
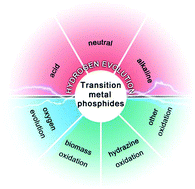
Transition metal phosphide (TMP) nanomaterials are recently considered to be versatile electrocatalysts with excellent activity and stability towards water splitting. This Frontier article will highlight recent advances in engineering the composition and structure of TMPs for higher electrochemical performances.
- This article is part of the themed collection: 2017 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...