 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoresponsive ruthenium-containing polymers: potential polymeric metallodrugs for anticancer phototherapy
Wen
Sun
,
Xiaolong
Zeng
and
Si
Wu
 *
*
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: wusi@mpip-mainz.mpg.de
First published on 16th November 2017
Abstract
This Frontier presents the recent development of photoresponsive Ru-containing polymers for cancer treatment. These novel Ru-containing polymers are prepared by introducing photoresponsive Ru complexes into polymers. Based on their chemical structures in aqueous solutions, these polymers can self-assemble into different nanostructures. The self-assembled nanostructures can circulate in the blood stream, accumulate at tumor tissue, and can be taken up by tumor cells. Red light, which can penetrate into tissue deeply, can induce the photodissociation of these polymers and sensitize singlet oxygen (1O2) generation. Both dissociated Ru complexes and generated 1O2 can inhibit the growth of tumor cells. Photoresponsive Ru-containing polymers provide a new platform for combined photodynamic therapy and photoactivated chemotherapy. The design strategies, self-assembly, photoresponsiveness, and anticancer effects of these polymers are introduced. Some remaining challenges for Ru-containing polymers for phototherapy are discussed.
Introduction
The design of new drugs for cancer treatment has been a major aspect in fundamental research.1,2 Metallodrugs play a vital role in this field. It is well known that the platinum complex, cisplatin, is the first US Food and Drug Administration (FDA) approved anticancer drug, which has been effectively used in clinics.2–4 However, cisplatin has side effects and suffers from drug resistance, which motivated researchers to develop new metallodrugs.5 As analogues of cisplatin, ruthenium (Ru) complexes are one of the good alternatives.1,2 To date, a large number of Ru complexes, which exhibit anticancer activities, have been designed.1,2,6 After uptake by cancer cells, Ru complexes can bind with DNA, which effectively block DNA and RNA synthesis, leading to programmed cell death.6–8 The increasing interest in Ru complexes is also due to their appealing photoresponsive properties.2,9–12 Some Ru complexes can generate singlet oxygen under light irradiation, which enables them to be photosensitizers for photodynamic therapy (PDT).10,13–15 In addition, light can uncage toxic Ru species or ligands from some Ru complexes for photoactivated chemotherapy (PACT).9,10 Therefore, some Ru complexes may be applied to combined PDT and PACT. We call them “phototherapy” in general in this Frontier.The use of photoresponsive Ru complexes for anticancer phototherapy has attracted increasing attention because combined PDT and PACT can improve therapeutic efficiency.1,2,9,10,13–15 The processes of using Ru complexes for phototherapy are similar to those of other photosensitive anticancer agents, such as photosensitizers or photoactivated (pro)drugs for PDT or PACT.16,17 First, photosensitive anticancer agents (e.g., Ru complexes) should be injected into the blood vessel. Then, the agents with appropriate properties could accumulate at the tumor site through blood circulation and internalize into cancer cells. Light irradiation at the tumor site will activate the Ru complexes to generate toxic species, and consequently, inhibit tumor growth.
Deep-tissue photoactivation of Ru complexes with red light
Light penetration into tissue is one of the preconditions for phototherapy.18 Many photoresponsive compounds, such as, o-nitrobenzyl, pyrene, and coumarin, have been studied and proposed for phototherapy.18,19 However, they are responsive to UV light or short wavelength visible light, which cannot penetrate deeply into tissue. In comparison, photoresponsive Ru complexes are promising for deep-tissue phototherapy because some of them are sensitive to red or near infrared (NIR) light in the “therapeutic window” (e.g., 650–900 nm).2,16,20 For example, our group synthesized four photoresponsive Ru complexes and studied the photoactivation of these complexes in deep tissue.21 Two of them (Ru3 and Ru4) have absorption tails in the “therapeutic window” and can be activated using red light (Fig. 1a). We demonstrated that 671 nm red light can activate Ru3 and Ru4 after passing through a 16 mm-thick tissue (Fig. 1b and c). Additionally, activated Ru4 caused the inhibition of cancer cells. These results suggested that red-light-responsive Ru complexes (e.g., Ru3 and Ru4) are promising for deep-tissue phototherapy. Photoresponsive Ru complexes are considered as promising agents for phototherapy and have been widely used in vitro.17 However, photoresponsive Ru complexes are problematic for in vivo applications. First, Ru complexes display poor bioavailability for cancer treatment. They can be rapidly cleared from the bloodstream, leading to inefficient accumulation at tumor sites.22 This problem could result in low therapeutic efficiency. Second, similar to other anticancer agents, Ru complexes may also cause serious toxic side effects on healthy tissues.1 Thus, improving the therapeutic efficiency of photoresponsive Ru complexes and reducing their side effects are currently two challenging problems.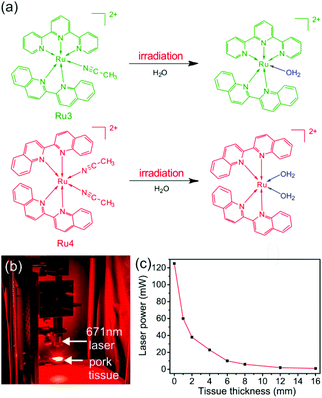 | ||
| Fig. 1 (a) Photoreactions of Ru3 and Ru4 under red light irradiation. (b) Photograph showing the laser (671 nm, 125 mW) passing through pork tissue. A laser spot can be observed after passing through the pork tissue. (c) Laser power after the laser (671 nm and 125 mW) passed through the pork tissue with different thicknesses. Reproduced with permission from ref. 21. Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
Photoresponsive Ru-containing polymers
To overcome the above-mentioned problems of low molecular weight Ru complexes, we designed Ru-containing block copolymers (BCPs) that could self-assemble into nanostructures in aqueous solutions.23,24 Photoresponsive Ru complexes are building blocks and functional moieties of the BCPs, which can be activated in tumor cells with light. Compared to low molecular weight Ru complexes, Ru-containing BCPs with proper self-assembled nanostructures and surface chemistry have longer blood circulation. In particular, nanoparticles with sizes of 10–200 nm show effective tumor accumulation through the enhanced permeability and retention (EPR) effect.25,26 Thus, self-assembled BCP nanoparticles may improve the bioavailability of photoresponsive Ru complexes and reduce their toxic side effects.Our group, for the first time, synthesized red-light-responsive Ru-containing BCPs and demonstrated their potential applications in phototherapy. Three side-chain Ru-containing BCPs (P1–P3) with different molecular weights were prepared (Fig. 2).23 Each BCP contained a hydrophilic and biocompatible poly(ethylene glycol) (PEG) block and a hydrophobic Ru-containing block. Depending on the molecular weight, the BCPs assembled into micelles, vesicles, and large compound micelles. All of the nanostructures could be taken up by cancer cells and the micelles showed the highest cellular uptake efficiency compared to the other two. The red-light irradiation of the BCPs released the anticancer Ru complexes and generated reactive 1O2 in cancer cells. The released anticancer Ru complex can be used for PACT and 1O2 can be used for PDT. The combined PDT and PACT showed enhanced anticancer activity. The cell viability decreased to 24%, 75% and 80% when cells were incubated with micelles, vesicles and large compounds, followed by 656 nm red light irradiation. The anticancer performance of micelles was better than those of vesicles and large compound micelles due to their more efficient cellular uptake. Moreover, under the dark conditions, there was no cleavage of the Ru complex from the BCP assemblies, suggesting that no side effect would occur in a normal microenvironment.
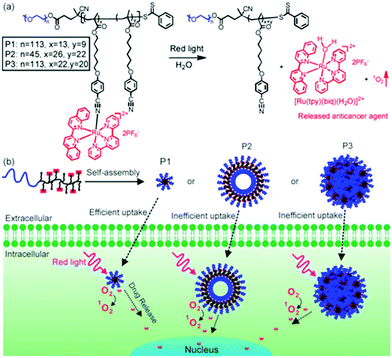 | ||
| Fig. 2 (a) Chemical structures of side-chain Ru-containing BCPs. Red light irradiation releases the Ru complex and generates singlet oxygen (1O2). P1, P2 and P3 are BCPs with different molecular weights. (b) Schematic illustration of the self-assembly of Ru-containing BCPs. P1, P2 and P3 self-assemble into micelles, hollow spheres and large compound micelles, which exhibit morphology-dependent cellular uptake and anticancer performance. Reproduced with permission from ref. 23. Copyright 2016 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
We also introduced Ru complexes into the main-chain of a BCP (Fig. 3).24 PolyRu is an ABA-type triblock copolymer that contains a hydrophobic and photolytic Ru-containing block and two hydrophilic and biocompatible PEG blocks (Fig. 3). The weight fraction of the Ru-containing block in PolyRu was more than 50%, demonstrating an efficient drug-loading capacity of the BCP. In an aqueous solution, PolyRu self-assembled into nanoparticles with an average diameter of 180 nm. The PolyRu nanoparticles could be effectively taken up by the cancer cells including HeLa, PC3, and HepG2. The photodegradation of PolyRu induced by 656 nm red light facilitated the release of anticancer Ru complexes, and consequently, the proliferation of cancer cells was efficiently inhibited. More importantly, in vivo experiments in a mouse model demonstrated that Ru-containing BCPs could accumulate at tumor sites and inhibit the growth of tumor under light irradiation. In addition, the nanoparticles did not cause any pathological tissue damage/abnormality during the treatment. Thus, phototherapy using Ru-containing BCPs eliminated systemic toxicity caused by Ru complexes in vivo.
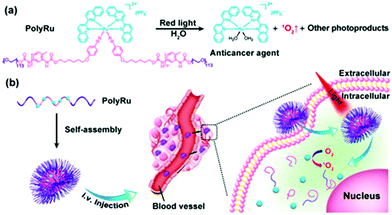 | ||
| Fig. 3 (a) Chemical structure of the main-chain Ru-containing BCP (PolyRu). Red light induces the degradation of PolyRu to generate the anticancer complex and 1O2. (b) Schematic illustration of self-assembly and phototherapy using PolyRu. Reproduced with permission from ref. 24. Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
Conclusions and outlook
In conclusion, photoresponsive Ru-containing BCPs have been developed for anticancer phototherapy. These BCPs self-assembled into nanoparticles which could circulate in the blood stream, accumulate at tumor tissue, and inhibit the growth of cancer cells under red-light irradiation. Both the released toxic Ru complexes and generated 1O2 exhibit the anticancer effect. More importantly, Ru-containing nanoparticles can improve the bioavailability of Ru complexes and reduce their toxic side effects in vivo. The design strategies described here can be used to develop new and improved Ru-containing polymers for anticancer phototherapy. The studies using photoresponsive Ru-containing polymers for biomedical applications have just started. There are some open questions and challenges regarding their synthesis, characterization, and applications. This field still awaits more exploration.First, from the chemistry point of view, finding ways to synthesize photoresponsive Ru-containing polymers with controlled molecular weights and architecture is a challenge. In our work, we either grafted Ru complexes to polymers (Fig. 2) or used polycondensation to polymerize Ru-containing monomers (Fig. 3). The “graft-to” method can only functionalize a part of the repeat units on the polymers. Polycondensation is difficult to control the molecular weight. Photo-responsive Ru-containing polymers, which are directly polymerized using controlled polymerization, have not been demonstrated.
Second, from the characterization point of view, it is difficult to analyze photoresponsive Ru-containing polymers using standard polymer analytical methods such as gel permeation chromatography (GPC) and static light scattering (SLS). The presence of charged moieties in such polymers may result in unreliable molecular weight characterization via GPC due to their interaction with the GPC column material.27 The measurement using SLS is also difficult because the light irradiation of the sample with an inappropriate wavelength may cause the dissociation of the polymers. Suitable characterization methods to analyze such polymers are expected to be conducted.
Third, from the therapeutic point of view, the anticancer efficiency of responsive Ru complexes needs further improvement. The Ru complexes used in the reported systems only demonstrated acceptable toxicity, which results in the moderate anticancer efficiency of these Ru-containing polymers. Developing polymers using high toxicity photoresponsive Ru complexes will help us improve the therapeutic efficacy of Ru-containing polymers.
Fourth, the activation wavelength of Ru-containing polymers needs to be further red-shifted. The best activation wavelength is at the NIR region. However, the absorption tails but not the peaks of the reported compounds just reach the NIR region. Although the two-photon process28,29 and upconversion-assisted photochemistry18,30–38 can activate Ru complexes using NIR light, both methods are based on inefficient non-linear optical processes. Two-photon absorption requires unsafe high-intensity laser excitation and only occurs at the focus of pulsed lasers. Thus, two-photon absorption is not suited for phototherapy applications. Upconversion-assisted photoactivation often requires a laser intensity of at least several hundred mW cm−2. Because light intensity decreases dramatically after passing through tissue (Fig. 1c), upconversion is problematic for deep-tissue applications. Thus, the direct red-shift of the activation wavelength of Ru-containing polymers to the NIR region is more promising for deep-tissue applications. For example, the use of fluorinated aromatic rings or compounds with high aromaticity may help to red shift the activation wavelength. Such Ru-containing polymers could be sensitive to low-intensity NIR light, which is suited for phototherapy in real applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
W. S. and X. Z. were supported by the CSC program. This work was supported by the Fonds der Chemischen Industrie (FCI, No. 661548) and the Deutsche Forschungsgemeinschaft (DFG, WU 787/2-1). Open Access funding was provided by the Max Planck Society.Notes and references
- L. Zeng, P. Gupta, Y. Chen, E. Wang, L. Ji, H. Chao and Z. S. Chen, Chem. Soc. Rev., 2017, 46, 5771–5804 RSC.
- C. Mari, V. Pierroz, S. Ferrari and G. Gasser, Chem. Sci., 2015, 6, 2660–2686 RSC.
- F. S. Mackay, J. A. Woods, P. Heringová, J. Kašpárková, A. M. Pizarro, S. A. Moggach, S. Parsons, V. Brabec and P. J. Sadler, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20743–20748 CrossRef CAS PubMed.
- N. P. E. Barry and P. J. Sadler, ACS Nano, 2003, 7, 5654–5659 CrossRef PubMed.
- V. Brabec and O. Novakova, Drug Resist. Updates, 2006, 9, 111–122 CrossRef CAS PubMed.
- J. D. Knoll and C. Turro, Coord. Chem. Rev., 2015, 282–283, 110–126 CrossRef CAS PubMed.
- E. Wachter, A. Zamora, D. K. Heidary, J. Ruiz and E. C. Glazer, Chem. Commun., 2016, 52, 10121–10124 RSC.
- R. E. Goldbach, I. Rodriguez-Garcia, J. H. van Lenthe, M. A. Siegler and S. Bonnet, Chem. – Eur. J., 2011, 17, 9924–9929 CrossRef CAS PubMed.
- J. D. Knoll, B. A. Albani and C. Turro, Acc. Chem. Res., 2015, 48, 2280–2287 CrossRef CAS PubMed.
- J. K. White, R. H. Schmehl and C. Turro, Inorg. Chim. Acta, 2017, 454, 7–20 CrossRef CAS PubMed.
- L. Zayat, C. Calero, P. Alborés, L. Baraldo and R. Etchenique, J. Am. Chem. Soc., 2003, 125, 882–883 CrossRef CAS PubMed.
- L. Zayat, O. Filevich, L. M. Baraldo and R. Etchenique, Philos. Trans. R. Soc., A, 2013, 371, 20120330 CrossRef PubMed.
- E. Wachter, D. K. Heidary, B. S. Howerton, S. Parkin and E. C. Glazer, Chem. Commun., 2012, 48, 9649–9651 RSC.
- H. Huang, B. Yu, P. Zhang, J. Huang, Y. Chen, G. Gasser, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2015, 54, 14049–14052 CrossRef CAS PubMed.
- N. W. Choi, S. S. Verbridge, R. M. Williams, J. Chen, J. Y. Kim, R. Schmehl, C. E. Farnum, W. R. Zipfel, C. Fischbach and A. D. Stroock, Biomaterials, 2012, 33, 2710–2722 CrossRef CAS PubMed.
- K. Liu, R. R. Xing, Q. L. Zou, G. H. Ma, H. Möhwald and X. H. Yan, Angew. Chem., Int. Ed., 2016, 55, 3036–3039 CrossRef CAS PubMed.
- M. Abbas, Q. L. Zou, S. K. Li and X. H. Yan, Adv. Mater., 2017, 29, 1605021 CrossRef PubMed.
- S. Wu and H. J. Butt, Adv. Mater., 2016, 28, 1208–1226 CrossRef CAS PubMed.
- J. F. Gohy and Y. Zhao, Chem. Soc. Rev., 2013, 42, 7117–7129 RSC.
- E. Wachter, B. S. Howerton, E. C. Hall, S. Parkin and E. C. Glazer, Chem. Commun., 2014, 50, 311–313 RSC.
- W. Sun, R. Thiramanas, L. D. Slep, X. Zeng, V. Mailander and S. Wu, Chem. – Eur. J., 2017, 23, 10832–10837 CrossRef CAS PubMed.
- W. P. R. J. H. Koch, F. P. Dwyer and E. C. Gyarfas, Aust. J. Biol. Sci., 1957, 10, 342 CrossRef.
- W. Sun, M. Parowatkin, W. Steffen, H.-J. Butt, V. Mailänder and S. Wu, Adv. Healthcare Mater., 2016, 5, 467–473 CrossRef CAS PubMed.
- W. Sun, S. Li, B. Haupler, J. Liu, S. Jin, W. Steffen, U. S. Schubert, H.-J. Butt, X.-J. Liang and S. Wu, Adv. Mater., 2017, 29, 1603702 CrossRef PubMed.
- J. Wang, K. Liu, R. R. Xing and X. H. Yan, Chem. Soc. Rev., 2016, 45, 5589–5604 RSC.
- Q. L. Zou, M. Abbas, L. Y. Zhao, S. K. Li, G. Z. Shen and X. H. Yan, J. Am. Chem. Soc., 2017, 139, 1921–1927 CrossRef CAS PubMed.
- M. A. R. Meier, B. G. G. Lohmeijer and U. S. Schubert, Macromol. Rapid Commun., 2003, 24, 852–857 CrossRef CAS.
- V. Nikolenko, R. Yuste, L. Zayat, L. M. Baraldo and R. Etchenique, Chem. Commun., 2005, 13, 1752–1754 RSC.
- V. San Miguel, M. Alvarez, O. Filevich, R. Etchenique and A. del Campo, Langmuir, 2012, 28, 1217–1221 CrossRef CAS PubMed.
- Z. Chen, W. Sun, H. J. Butt and S. Wu, Chem. – Eur. J., 2015, 21, 9165–9170 CrossRef CAS PubMed.
- S. He, K. Krippes, S. Ritz, Z. Chen, A. Best, H. J. Butt, V. Mailänder and S. Wu, Chem. Commun., 2015, 51, 431–434 RSC.
- Z. Chen, S. He, H. J. Butt and S. Wu, Adv. Mater., 2015, 27, 2203–2206 CrossRef CAS PubMed.
- S. H. Askes, A. Bahreman and S. Bonnet, Angew. Chem., Int. Ed., 2014, 53, 1029–1033 CrossRef CAS PubMed.
- S. H. Askes, M. Kloz, G. Bruylants, J. T. Kennis and S. Bonnet, Phys. Chem. Chem. Phys., 2015, 17, 27380–27390 RSC.
- Z. Chen, Y. Xiong, R. Etchenique and S. Wu, Chem. Commun., 2016, 52, 13959–13962 RSC.
- S. Wu, J. P. Blinco and C. Barner-Kowollik, Chem. – Eur. J., 2017, 23, 8325–8332 CrossRef CAS PubMed.
- S. Wu and H.-J. Butt, Phys. Chem. Chem. Phys., 2017, 19, 23585–23596 RSC.
- Z. Chen, R. Thiramanas, M. Schwendy, C. Xie, S. H. Parekh, V. Mailänder and S. Wu, Small, 2017, 1700997 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |
