Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A new family of clusters containing a silver-centered tetracapped [Ag@Ag4(μ3-P)4] tetrahedron, inscribed within a N12 icosahedron†
Alexander V.
Artem'ev
 *a,
Irina Yu.
Bagryanskaya
bc,
Evgeniya P.
Doronina
d,
Peter M.
Tolstoy
*a,
Irina Yu.
Bagryanskaya
bc,
Evgeniya P.
Doronina
d,
Peter M.
Tolstoy
 e,
Artem L.
Gushchin
ac,
Mariana I.
Rakhmanova
a,
Alexander Yu.
Ivanov
e and
Anastasiya O.
Suturina
d
e,
Artem L.
Gushchin
ac,
Mariana I.
Rakhmanova
a,
Alexander Yu.
Ivanov
e and
Anastasiya O.
Suturina
d
aNikolaev Institute of Inorganic Chemistry, Siberian Branch of Russian Academy of Sciences, 3, Akad. Lavrentiev Ave., Novosibirsk 630090, Russia. E-mail: chemisufarm@yandex.ru
bN. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of Russian Academy of Sciences, 9, Akad. Lavrentiev Ave., Novosibirsk 630090, Russia
cNovosibirsk State University, National Research University, Department of Natural Sciences, 2, Pirogova Str., Novosibirsk 630090, Russia
dA. E. Favorsky Irkutsk Institute of Chemistry, Siberian Branch of the Russian Academy of Sciences, 1 Favorsky Str., 664033 Irkutsk, Russia
eSt. Petersburg State University, Center for Magnetic Resonance, Universitetskij pr. 26, St. Petersburg 198504, Russia
First published on 24th August 2017
Abstract
An unprecedented silver-centered P-tetracapped [Ag@Ag4(μ3-P)4] tetrahedron inscribed within a N12 icosahedral cage has been discovered in the novel family of luminescent clusters. The latter are easily self-assembled by reacting AgI salts with tris(2-pyridyl)phosphine (Py3P).
Currently, silver(I) complexes and clusters attract considerable attention because of their intriguing structural, photophysical, catalytic and biological properties.1 One of the most powerful approaches to the synthesis of well-defined silver(I) clusters is self-assembly2 from Ag+ ions and diverse ligands such as N-heterocycles,3 carboxylates,4 sulfides and selenides,5 1,1-dithiolates,6 and alkynyl anions.7 Among a plethora of ligands that are used for this purpose, phosphines containing additional donor sites are of special interest because they allow assembling a number of unique architectures.8 For instance, based on diphenyl-2-pyridylphosphine (dppy), a series of highly luminescent heterometallic Ag/Au clusters have been synthesized, e.g. [Au3(E)Ag(dppy)3]2+,9 [(CCN)2Au8Ag4(dppy)8(MeCN)2]6+,10 and [RCOCAu4Ag4(dppy)4]5+.11 Recently,12 using bis(2-pyridyl)phenylphosphine, the original 1D polymer [Ag2(PPhPy2)2⋯Cl−⋯Ag2(PPhPy2)2]n has been designed. Also, it is pertinent to note that silver(I) complexes with pyridylphosphines are prospective antitumour agents.13
Herein, we report on a new family of luminescent Ag(I) clusters with an unprecedented core structure, which can be viewed as a silver-centered phosphorus-tetracapped tetrahedron [Ag@Ag4(μ3-P)4], inscribed within a N12 icosahedral cage. These clusters have been readily self-assembled by reacting silver(I) salts with tris(2-pyridyl)phosphine under mild conditions. The photophysical and electrochemical properties as well as the electronic structure of the novel complexes have also been investigated.
We have discovered that tris(2-pyridyl)phosphine easily reacts with AgOTf in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio (room temperature, CH2Cl2/MeCN, 1 h) to afford the unprecedented cluster [Ag@Ag4(Py3P)4(OTf)4](OTf) (1) in 84% yield (the synthetic details are in the ESI†). It is noteworthy that the variation of the reactant molar ratio (e.g. Ag/L = 1
5 molar ratio (room temperature, CH2Cl2/MeCN, 1 h) to afford the unprecedented cluster [Ag@Ag4(Py3P)4(OTf)4](OTf) (1) in 84% yield (the synthetic details are in the ESI†). It is noteworthy that the variation of the reactant molar ratio (e.g. Ag/L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 2
2 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
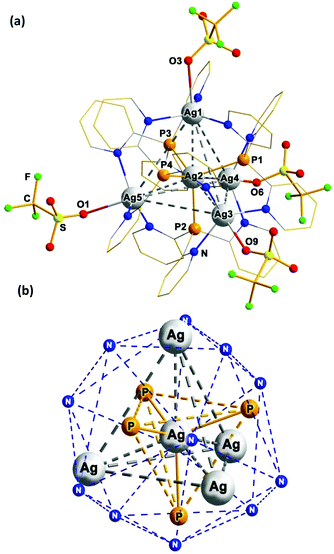
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) does not lead to the formation of other clusters apart from 1. X-Ray diffraction (XRD) analysis reveals that this compound crystallizes from acetone solution as the 1·4Me2CO solvate. The [Ag@Ag4(Py3P)4(OTf)4]+ cation of the latter (Fig. 1a) contains a structurally remarkable [Ag@Ag4(μ3-P)4] skeleton which is best described as a C3-symmerical Ag-centered Ag4 tetrahedron (τ4 = 0.954 (ref. 14)) whose each triangular face is capped by a phosphorus atom (μ3-P) of the Py3P ligands (Fig. 1b). Overall, the [Ag@Ag4(μ3-P)4] core is inscribed within a N12 icosahedron, constituted by the twelve pyridine nitrogen atoms of four Py3P ligands. The central Ag atom has a slightly distorted P4 tetrahedral environment (τ4 = 0.984 (ref. 14)), constituted by the four P atoms of the Py3P ligands [Ag–P, 2.5766(16)–2.6095(15) Å]. Each vertex Ag atom shows three short bonds with the N atoms of the three neighbouring Py3P ligands [Ag–N, 2.272(5)–2.306(5) Å] and a one longer bond with a triflate O atom, thus adopting a [3 + 1] trigonal pyramidal geometry. The three Ag–O bond lengths are similar (ca. 2.620 Å), which suggests a weak association of the triflate anions with the Ag3, Ag4 and Ag5 atoms, whereas the Ag1–O3 distance [2.7400(53) Å] is clearly non-bonded. The Agcentral–Agvertex distances are quite short ranging from 3.0917(7) to 3.1638(6) Å {cf. with twice the van der Waals radius of Ag, 3.4 Å}. Meanwhile, the Ag–Ag distances along the edges of the Ag4 tetrahedron are too long [5.0031(7)–5.2553(7) Å] for any argentophilic interactions. Thus, each Py3P ligand binds the central metal (via the P atom) and the three adjacent Agvertex ions (via pyridine N donors), exhibiting a P,N,N′,N′′-coordination pattern.
3) does not lead to the formation of other clusters apart from 1. X-Ray diffraction (XRD) analysis reveals that this compound crystallizes from acetone solution as the 1·4Me2CO solvate. The [Ag@Ag4(Py3P)4(OTf)4]+ cation of the latter (Fig. 1a) contains a structurally remarkable [Ag@Ag4(μ3-P)4] skeleton which is best described as a C3-symmerical Ag-centered Ag4 tetrahedron (τ4 = 0.954 (ref. 14)) whose each triangular face is capped by a phosphorus atom (μ3-P) of the Py3P ligands (Fig. 1b). Overall, the [Ag@Ag4(μ3-P)4] core is inscribed within a N12 icosahedron, constituted by the twelve pyridine nitrogen atoms of four Py3P ligands. The central Ag atom has a slightly distorted P4 tetrahedral environment (τ4 = 0.984 (ref. 14)), constituted by the four P atoms of the Py3P ligands [Ag–P, 2.5766(16)–2.6095(15) Å]. Each vertex Ag atom shows three short bonds with the N atoms of the three neighbouring Py3P ligands [Ag–N, 2.272(5)–2.306(5) Å] and a one longer bond with a triflate O atom, thus adopting a [3 + 1] trigonal pyramidal geometry. The three Ag–O bond lengths are similar (ca. 2.620 Å), which suggests a weak association of the triflate anions with the Ag3, Ag4 and Ag5 atoms, whereas the Ag1–O3 distance [2.7400(53) Å] is clearly non-bonded. The Agcentral–Agvertex distances are quite short ranging from 3.0917(7) to 3.1638(6) Å {cf. with twice the van der Waals radius of Ag, 3.4 Å}. Meanwhile, the Ag–Ag distances along the edges of the Ag4 tetrahedron are too long [5.0031(7)–5.2553(7) Å] for any argentophilic interactions. Thus, each Py3P ligand binds the central metal (via the P atom) and the three adjacent Agvertex ions (via pyridine N donors), exhibiting a P,N,N′,N′′-coordination pattern.
The [Ag@Ag4(Py3P)4] core of 1 is chiral due to a propeller-like arrangement of the pyridine cycles around four vertex silver atoms. Interestingly enough, exclusively ΔΔΔΔ- and ΛΛΛΛ-helical isomers are found in the packing of 1 (Fig. S1†), i.e. the Agvertex atoms in each stereoisomer have an identical configuration.
Encouraged by the first result, we next have examined whether the disclosed approach could be used for the assembly of similar aggregates from other silver(I) salts. The experiments have shown that [Ag(MeCN)4]BF4 and [Ag(MeCN)4]PF6 interact with Py3P in the same way to afford structurally related clusters, [Ag4(Ag)(Py3P)4](BF4)5 (2) and [Ag4(Ag)(Py3P)4](PF6)5 (3), in 99 and 86% yield, respectively.† The data of elemental analysis, 1H NMR and FT-IR spectroscopy of samples 2 and 3 confirm the above-mentioned formulations. The diffusion of hexane into a solution of 2 in an acetone/MeCN mixture results in X-ray quality crystals, the asymmetric unit of which consists of two cations, [Ag@Ag4(Py3P)4(Me2CO)(MeCN)3]5+ (Fig. 2a) and suchlike [Ag@Ag4(Py3P)4(Me2CO)(MeCN)2]5+ (Fig. S2†) along with ten BF4− anions. Similarly, the equal number of ΔΔΔΔ- and ΛΛΛΛ-enantiomers for both the cations is presented in the centrosymmetric crystals of 2. On the whole, the [Ag@Ag4(Py3P)4] skeleton 2 is almost superimposable with that of 1 (see the overlay of these fragments in Fig. S4†). One of the Agvertex atoms of 2 is additionally ligated by O from acetone, whereas the remaining Agvertex atoms are weakly bonded with MeCN molecules (Fig. 2a and S2†). At that, two basal atoms, Ag8 and Ag9, have quite similar Ag–N distances (2.542 and 2.550 Å), whereas the corresponding bond for Ag6 is somewhat longer: 2.681 Å.
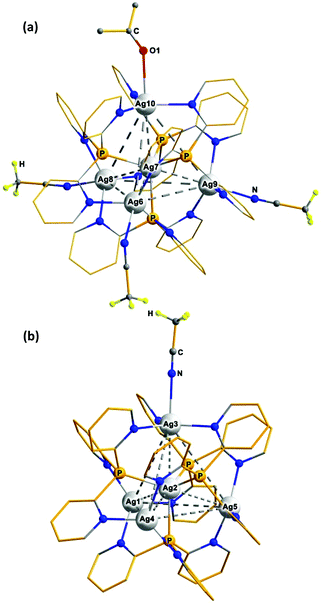 | ||
| Fig. 2 The structures of the [Ag@Ag4(Py3P)4(Me2CO)(MeCN)3]5+ (a) and [Ag@Ag4(Py3P)4(MeCN)]5+ (b) cations in 2·Me2CO·2.5MeCN and 3·MeCN, correspondingly (the pyridine H atoms are omitted for clarity). | ||
The crystals of [Ag@Ag4(Py3P)4(MeCN)](PF6)5 (3·MeCN) have been obtained by diffusion of diethyl ether into an MeCN solution of 3. XRD analysis revealed (Fig. 2b) that the [Ag@Ag4(Py3P)4] unit in [Ag@Ag4(Py3P)4(MeCN)]5+ is nearly the same as that in 1 (Fig. S5†). As in the case in 2, the PF6− anions of 3 remain non-coordinated. Therefore, the three basal Ag atoms are saturated by pyridyl N donors in a trigonal planar manner, while the fourth Ag centre (Ag3) is additionally ligated by a MeCN solvent molecule (Ag–N 2.497 Å).
Significantly, using two different Ag(I) salts in the reaction with Py3P allows [Ag@Ag4(Py3P)4] clusters bearing two types of counterions to be synthesized. So, we have found that [Ag(MeCN)4]PF6 and four equivalents of [Ag(MeCN)4]BF4 may be easily self-assembled into the pentanuclear cluster [Ag4(Ag)(Py3P)4](PF6)(BF4)4 (4) in 85% yield by the addition of four equivalents of Py3P.† From the MeCN/Et2O system, cluster 4 crystallizes as solvate 4·6MeCN containing [Ag@Ag4(Py3P)4(MeCN)4]5+ cations (Fig. 3), the core of which is comparable to that of 1–3 (Fig. S6†).
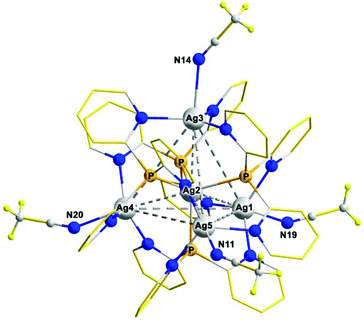 | ||
| Fig. 3 The structures of the [Ag@Ag4(Py3P)4(MeCN)4]5+ cation in 4·6MeCN, correspondingly (the pyridine H atoms are omitted for clarity). | ||
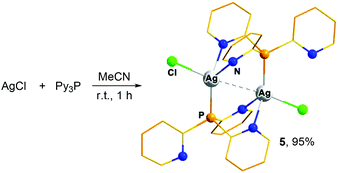
Our attempts to obtain a similar polynuclear cluster using AgCl in reaction with Py3P in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio lead to the isolation of the dinuclear complex [Ag(Cl)(μ-Py3P)]2 (5) in quantitative yield (Scheme 1, the synthetic details, and structural and luminescence properties are given in the ESI†). According to the XRD study, two Ag atoms in 5 are bridged by two Py3P ligands in a head-to-tail fashion so that each metal coordinates two N atoms and one P atom form two adjacent ligands (Scheme 1 and Fig. S3†). Note that complexes of a similar structure, but with the PhPy2P ligand, were previously described.12,15 The Ag–Ag distances in both independent molecules of 5 [3.2615(8) and 3.2701(9) Å] imply argentophilic interactions. In the solid state, this cluster exhibits blue-green emission (λmax = 500 nm, Fig. S30†) with the modest PLQY (8% at 298 K).†
4 molar ratio lead to the isolation of the dinuclear complex [Ag(Cl)(μ-Py3P)]2 (5) in quantitative yield (Scheme 1, the synthetic details, and structural and luminescence properties are given in the ESI†). According to the XRD study, two Ag atoms in 5 are bridged by two Py3P ligands in a head-to-tail fashion so that each metal coordinates two N atoms and one P atom form two adjacent ligands (Scheme 1 and Fig. S3†). Note that complexes of a similar structure, but with the PhPy2P ligand, were previously described.12,15 The Ag–Ag distances in both independent molecules of 5 [3.2615(8) and 3.2701(9) Å] imply argentophilic interactions. In the solid state, this cluster exhibits blue-green emission (λmax = 500 nm, Fig. S30†) with the modest PLQY (8% at 298 K).†
The pentanuclear clusters obtained are air- and light-stable powders and show good solubility in acetonitrile. All these clusters exhibit a high stability in solutions and can be recrystallized from them. The ESI-mass spectra of the clusters exhibit a number of low intensive peaks assigned to polynuclear Ag-containing species, thus indicative of a fragmentation of the [Ag4(Ag)(Py3P)4]5+ cation under ESI-conditions.
The NMR data reveal that the cluster cations of 1–4 remain intact in the solution state. So, in the 31P{1H} NMR spectrum of 1 dissolved in CD3CN at 298 K, only one signal is visible at ca. 14.6 ppm with a large doublet splitting due to the 31P–109/107Ag coupling. The magnitude of this coupling (238 Hz for 31P–109Ag and 207 Hz for 31P–107Ag) clearly suggests that all four P atoms are bonded to the Ag atom.16 Moreover, couplings with other Ag atoms of the complex are resolved as well (see. Fig. 4, top, for the spectra and assignment of the couplings; we have tentatively assigned all 6–7 Hz splittings to the 2J(P–Ag–Ag) couplings, assuming that they are similar in value and that the 3J(P–C–N–Ag) coupling is too small to be resolved). The high resolution constant time 1H,15N-HMBC spectrum, shown in Fig. 4, bottom, further reveals the 1J(15N,109/107Ag) and 2J(15N,109/107Ag) couplings consistent with the structure of the complex, as well as the 2J(31P,15N) coupling of ca. 56 Hz. Note that within the spectral line width the differences in couplings to 109Ag and 107Ag are not always resolved. The complete set of 1H, 31P{1H}, 109Ag and lower resolution 1H,15N-HMBC NMR spectra for complexes 1, 2, 3 and 4 dissolved in CD3CN is given in the ESI (Fig. S7–S22†). The 109Ag NMR signals of 1–4 do not show clear Ag–P splitting patterns. However, these signals have similar line shapes with a pedestal (see Fig. S9, S13, S17 and S21†) indicating the presence of some kind of splitting. At the given signal-to-noise ratio (up to 4 days of acquisition), it is hard to speculate about the number of signals and the types of spin systems. In turn, the signal of the central silver atom is not observed in the 109Ag NMR spectra because it should be four times less intensive and additionally split into complicated multiplets due to the one-bond couplings with phosphorous and silver nuclei.
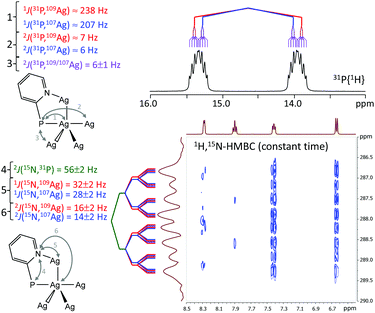 | ||
| Fig. 4 31P{1H} (top) and constant time 1H,15N-HMBC (bottom) NMR spectra of 1 dissolved in CD3CN at 300 K. | ||
The UV-Vis spectra of clusters 1–4 are characterized by a strong band at 260 nm and a weak shoulder expanding from 275 to 340 nm (Fig. S29†). The high-energy band could be assigned to intraligand transitions because the Py3P itself displays the same absorption. The weak band in the region 275–340 nm is tentatively assigned to metal-to-ligand charge transfer transitions that is in accordance with DFT calculations (vide infra).
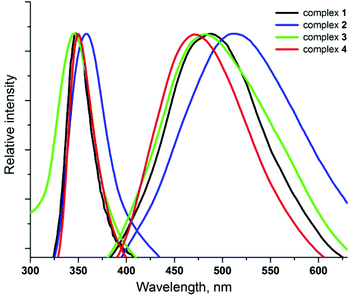
The luminescence properties of clusters 1–4 have been preliminarily investigated in the solid state. All these exhibit a greenish-blue emission at room temperature with the quantum yields up to 24% (Table 1). The excitation and emission spectra of 1–4 are depicted in Fig. 5. Clusters 4, 3, 1 and 2 show a broad emission with the maxima at 470, 480, 490 and 510 nm, respectively, i.e. the distinct red-shift of the emission maxima is observed in this range, while the free Py3P displays a broad emission with λmax at about 420 nm. Taking into account the literature data,3c the origin of luminescence of 1–4 can be ascribed to metal-to-ligand charge transfer states, perturbed by the presence of Ag⋯Ag interactions.
| λ exmax (nm) | λ emmax (nm) | ϕ PL (%) | |
|---|---|---|---|
| 1 | 350 | 490 | 14 |
| 2 | 360 | 510 | 24 |
| 3 | 350 | 480 | 18 |
| 4 | 350 | 470 | 2 |
The redox properties of clusters 1–4 have been investigated by cyclic voltammetry (CV). All compounds demonstrate similar redox behavior in the negative range (up to −2 V) and reveal irreversible reduction processes which can be assigned to the AgI/Ag0 couple. The cathodic potential values (Table S3†) strongly depend on the number of scans and dramatically change on going from the first scan to the second one. In particular, the CV of 1 shows two cathodic peaks at −1.2 and −1.5 V (vs. Ag/AgCl) at the first scan which are further transformed to the broad wave centered at −0.77 V at the second scan (Fig. 6). This behavior can be explained by absorption of the reduction products on the surface of the working electrode.
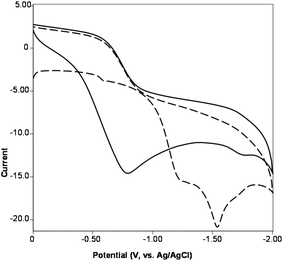 | ||
| Fig. 6 CV of 1 in CH3CN between 0 ↔ −2 V at 0.1 V s−1 scan rate (dashed line is the first scan, solid line is the second scan). | ||
Conclusions
In summary, we have presented the novel family of luminescent clusters containing a remarkable Ag-centered P-tetracapped tetrahedral [Ag@Ag4(μ3-P)4] unit inscribed within a N12 icosahedron. Taking into account the intriguing structure of these clusters and their facile and almost quantitative self-assembly from available reagents, one expects that the reported finding would stimulate intense research interest in this area. Further investigation of the silver clusters is in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Russian Foundation for Basic Research (Grant 15-03-05591). We also are grateful to Multi-Access Chemical Service Center SB RAS for XRD analysis. Supported by Russian Ministry of Science and Education under 5-100 Excellence Programme.Notes and references
- (a) G. Fang and X. Bi, Chem. Soc. Rev., 2015, 44, 8124–8173 RSC; (b) S. Medici, M. Peana, G. Crisponi, V. M. Nurchi, J. I. Lachowicz, M. Remelli and M. A. Zoroddu, Coord. Chem. Rev., 2016, 327–328, 349–359 CrossRef CAS; (c) H. Pellissier, Chem. Rev., 2016, 116, 14868–14917 CrossRef CAS PubMed.
- S.-L. Zheng, M.-L. Tong and X.-M. Chen, Coord. Chem. Rev., 2003, 246, 185–202 CrossRef CAS.
- (a) A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouga, N. V. Zyk and M. Schröder, Coord. Chem. Rev., 2001, 222, 155–192 CrossRef CAS; (b) P. J. Steel and C. M. Fitchett, Coord. Chem. Rev., 2008, 252, 990–1006 CrossRef CAS; (c) E. M. Njogu, B. Omondi and V. O. Nyamori, J. Coord. Chem., 2015, 68, 3389–3431 CrossRef CAS; (d) X.-P. Wang, T.-P. Hu and D. Sun, CrystEngComm, 2015, 17, 3393–3417 RSC.
- (a) L. Brammer, M. D. Burgard, C. S. Rodger, J. K. Swearingen and N. P. Rath, Chem. Commun., 2001, 2468–2469 RSC; (b) Q.-M. Wang and T. C. W. Mak, J. Am. Chem. Soc., 2001, 123, 7594–7600 CrossRef CAS PubMed.
- (a) O. Fuhr, S. Dehnen and D. Fenske, Chem. Soc. Rev., 2013, 42, 1871–1906 RSC; (b) Y.-P. Xie, J.-L. Jin, G.-X. Duan, X. Lu and T. C. W. Mak, J. Coord. Chem., 2017, 331, 54–72 CrossRef CAS.
- (a) J.-H. Liao, C. Latouche, B. Li, S. Kahlal, J.-Y. Saillard and C. W. Liu, Inorg. Chem., 2014, 53, 2260–2267 CrossRef CAS PubMed; (b) R. S. Dhayal, J.-H. Liao, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2015, 54, 3702–3706 CrossRef CAS PubMed; (c) R. S. Dhayal, Y.-R. Lin, J.-H. Liao, Y.-J. Chen, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Chem. – Eur. J., 2016, 22, 9943–9947 CrossRef CAS PubMed.
- (a) Q.-M. Wang, Y.-M. Lin and K.-G. Liu, Acc. Chem. Res., 2015, 48, 1570–1579 CrossRef CAS PubMed; (b) Y.-T. Chen, I. S. Krytchankou, A. J. Karttunen, E. V. Grachova, S. P. Tunik, P.-T. Chou and I. O. Koshevoy, Organometallics, 2017, 36, 480–489 CrossRef CAS.
- (a) M. Scheer, L. J. Gregoriades, A. V. Virovets, W. Kunz, R. Neueder and I. Krossing, Angew. Chem., Int. Ed., 2006, 45, 5689–5693 CrossRef CAS PubMed; (b) S. L. James, Chem. Soc. Rev., 2009, 38, 1744–1758 RSC; (c) R. Meijboom, R. J. Bowen and S. J. Berners-Price, Coord. Chem. Rev., 2009, 253, 325–342 CrossRef CAS.
- (a) Q.-M. Wang, Y.-A. Lee, O. Crespo, J. Deaton, C. Tang, H. J. Gysling, M. C. Gimeno, C. Larraz, M. D. Villacampa, A. Laguna and R. Eisenberg, J. Am. Chem. Soc., 2004, 126, 9488–9489 CrossRef CAS PubMed; (b) J.-H. Jia and Q.-M. Wang, J. Am. Chem. Soc., 2009, 131, 16634–16635 CrossRef CAS PubMed; (c) L.-Q. Mo, J.-H. Jia, L. Sun and Q.-M. Wang, Chem. Commun., 2012, 48, 8691–8693 RSC.
- X.-L. Pei, Y. Yang, Z. Lei and Q.-M. Wang, J. Am. Chem. Soc., 2013, 135, 6435–6437 CrossRef CAS PubMed.
- X.-L. Pei, Y. Yang, Z. Lei, S.-S. Chang, Z.-J. Guan, X.-K. Wan, T.-B. Wen and Q.-M. Wang, J. Am. Chem. Soc., 2015, 137, 5520–5525 CrossRef CAS PubMed.
- T. Zhang, C. Ji, K. Wang, D. Fortin and P. D. Harvey, Inorg. Chem., 2010, 49, 11069–11076 CrossRef CAS PubMed.
- (a) S. J. Berners-Price, R. J. Bowen, P. J. Harvey, P. C. Healy and G. A. Koutsantonis, J. Chem. Soc., Dalton Trans., 1998, 1743–1750 RSC; (b) J. J. Liu, P. Galettisa, A. Farr, L. Maharaja, H. Samarasinha, A. C. McGechan, B. C. Baguley, R. J. Bowen, S. J. Berners-Price and M. J. McKeage, J. Inorg. Biochem., 2008, 102, 303–310 CrossRef CAS PubMed.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- O. Crespo, M. C. Gimeno, A. Laguna and C. Larraz, Z. Naturforsch., B: Chem. Sci., 2009, 64, 1525–1534 CrossRef CAS.
- (a) S. J. B. Price, C. Brevard, A. Pagelot and P. J. Sadler, Inorg. Chem., 1985, 24, 4278–4281 CrossRef CAS; (b) A. D. Zotto and E. Zangrando, Inorg. Chim. Acta, 1998, 277, 111–117 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterization data, NMR and FT-IR spectra, photophysical and electrochemical data, computation details. CCDC 1472281, 1472282, 1532341 and 1538903. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt02597a |
| This journal is © The Royal Society of Chemistry 2017 |