Synthesis of a Ni2P/Ni12P5 bi-phase nanocomposite for the efficient catalytic reduction of 4-nitrophenol based on the unique n–n heterojunction effects†
Abstract
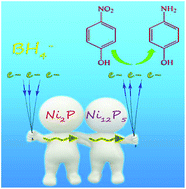
A novel heterostructure catalyst of Ni2P/Ni12P5 has been fabricated through a simple solvothermal method by modifying the molar ratio of the initial raw materials. The products are characterized by X-ray powder diffraction (XRD), field emission scanning electron microscopy (FE-SEM), high-resolution transmission electron microscopy (HRTEM), nitrogen adsorption and X-ray photoelectron spectroscopy (XPS). It is found that the two phases, Ni2P and Ni12P5, are interlaced with one another in the as-formed nanocomposite, resulting in more interfaces. The bi-phase catalyst exhibits a markedly enhanced catalytic activity in the reduction of 4-nitrophenol, as compared to that of single Ni2P or Ni12P5. The enhanced catalytic activity can be attributed to the unique n–n series effects, which result in the increased ease of electron transfer over the Ni2P/Ni12P5 bi-phase catalyst.


 Please wait while we load your content...
Please wait while we load your content...