Open Access Article
Open Access ArticleRing opening polymerisation of lactide with uranium(IV) and cerium(IV) phosphinoaryloxide complexes†
Fern
Sinclair
,
Johann A.
Hlina‡
,
Jordann A. L.
Wells
,
Michael P.
Shaver
 * and
Polly L.
Arnold
* and
Polly L.
Arnold
 *
*
EaStCHEM School of Chemistry, Joseph Black Building, University of Edinburgh, Edinburgh EH9 3FJ, UK. E-mail: polly.arnold@ed.ac.uk; michael.shaver@ed.ac.uk
First published on 2nd August 2017
Abstract
The C3-symmetric uranium(IV) and cerium(IV) complexes Me3SiOM(OArP)3, M = U (1), Ce (2), OArP = OC6H2-6-tBu-4-Me-2-PPh2, have been prepared and the difference between these 4f and 5f congeners as initiators for the ring opening polymerisation (ROP) of L-lactide is compared. The poorly controlled reactivity of the homoleptic analogue U(OArP)4 (3) demonstrates the importance of the M-OSiMe3 initiating group. The incorporation of a nickel atom in 1 to form the U–Ni heterobimetallic complex Me3SiOU(OArP)3Ni (4) may be the first example of the use of the inverse trans influence to switch the reactivity of a complex. This would imply the formation of the U–Ni bond strengthens the U–OSiMe3 bond to such an extent that the ROP catalysis is switched off. Changing the conditions to immortal polymerisation dramatically increases polymerisation rates, and switches the order, with the Ce complex now faster than the U analogue, suggesting ligand protonolysis to afford a more open coordination sphere. For the ROP of rac-lactide, uranium complex 1 promotes heterotacticity at the highest levels of stereocontrol yet reported for an actinide complex.
Introduction
Poly(lactic acid), PLA, is a now common-place biodegradable polyester built from the renewable cyclic diester lactide. With a monomer feedstock readily derived from resources such as corn and sugarbeets, PLA is playing an increasingly important role as a sustainable alternative in plastic packaging and modern biomaterials.1–3 While the thermal and mechanical properties of PLA can be dramatically changed by tuning molecular weights, blending with other polymers, or building more complicated macrostructures, the stereochemical control over the opening of D, L, or meso monomers has perhaps the most dramatic impact. Thus, catalyst design to control this tacticity in ring-opening polymerisations (ROP) of lactide and other cyclic esters is at the forefront of research at the inorganic/polymer interface.While industrial PLA production is dominated by an unselective tin-mediated reaction,1 a wide range of Lewis acidic metal complexes can facilitate either isotactic or heterotactic ROP (vide infra). Importantly, the design principles that guide how a ligand will govern tacticity are still not clear.4 One ligand family of particular interest are the C3 tris(phenolate)trianions; stereoselective initiators based on ZrIV have generated highly heterotactic polymers,5 while we have previously shown that a chiral, racemic heterobidentate alkoxide can selectively assemble to form homochiral, C3 symmetric complexes that are active initiators for the formation of isotactic PLA (Pi = 0.75, 298 K).6
Although often overlooked due to misconceptions of scarcity and excessive oxophilicity, the f-block cations possess a unique capability to tune the ROP performance due to the available range of size and Lewis acidity.7–10 This variability is difficult for other metals; the propensity of InIII to adopt a coordination number of 5 limited the scope in our system.11 While rare, both CeIII and CeIV are known to initiate ROP reactions, and judicious choice of ligands can dramatically alter activity. For instance, the CeIII initiator Ce(OtBu)(phosfen) (phosfen = 1,1′-di(2-t-butyl-6-diphenyl-phosphiniminophenoxy)ferrocene) is a fast initiator for polymerisation, exhibiting good control of Đ.12
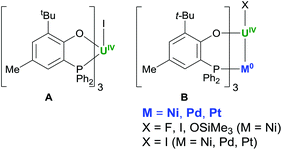
We recently demonstrated how the simple heterobidentate O–P aryloxide anion [OC6H2-6-tBu-4-Me-2-PPh2]− (OArP) is an excellent hemilabile ligand for UIV, with only weak P-coordination to the U centre in UI(OArP)3A (Chart 1).13
The strongly bound, sterically protected U–OAr groups help enforce a C3-symmetry on the complexes when a second, softer metal cation is added to bind the three P donors, forming XUIV(OArP)3MOB, for which the U–Ni bond is the strongest. We reasoned that the C3-symmetry available to the UIV complex, and the tunability provided by formation of a bonding interaction with the added M in the lower pocket, might afford good control over the ROP of lactide, while the hemilabile ligand framework would allow us to probe the important coordination chemistry factors that shape both polymerisation activity and tacticity control.
Herein we describe the preparation and comparison of new C3-symmetric UIV and CeIV initiators which offer surprising reactivity differences under living and immortal polymerisation conditions, and additionally explore the effect of the incorporation of a transition metal on this reactivity (Fig. 1).
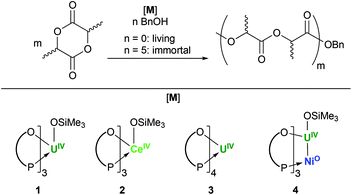 | ||
| Fig. 1 Summary of ROP of L-lactide studied for the new UIV and CeIV initiators 1 to 4. The O–P arc is used to represent the OArP ligand (OArP = OC6H2-6-tBu-4-Me-2-PPh2-κ2O,P). | ||
Results and discussion
Synthesis
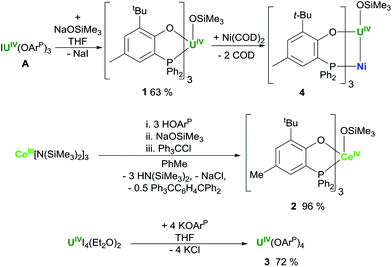
The uranium complex Me3SiOU(OArP)31 is prepared by treatment of IU(OArP)3 with equimolar NaOSiMe3 in THF solution (Scheme 1). The methodology is analogous to that we used to prepare B for M = Ni, X = OSiMe3 (Chart 1), which is referred to here as Me3SiOU(OArP)3Ni 4.13 The homoleptic uranium complex U(OArP)43 is prepared from the reaction of UI4(Et2O)2 with four molar equivalents of KOArP in THF.The cerium Me3SiOCe(OArP)32 complex is not conveniently made by salt metathesis routes, but in a one-pot procedure the cerium(III) tris(amide) Ce[N(SiMe3)2]3 reacts with three equivalents of HOArP to form Ce(OArP)3 which is further functionalised and then oxidised in situ by sequential addition of NaOSiMe3 and trityl chloride, yielding 2 as dark brown crystals in 96% (relative to the cerium amide), and the group 1 halide and Gomberg's dimer as by-products, Scheme 1.14,15
Crystallography
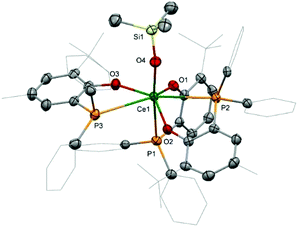
A sample of 2 suitable for X-ray diffraction was grown from a concentrated hexane solution (Fig. 2). The seven-coordinate cerium centre is tethered to the ligands with Ce–O distances from 2.190(2) to 2.162(2) Å for the aryloxides down to 2.067(2) Å for the trimethylsiloxide. The diversity in the Ce–P distances of 3.1575(7), 3.190(1), and 3.307(1) Å may be attributed to the minimisation of steric interactions between the large ligands at the expense of the weaker, more labile Ce–P interaction.16 A single crystal of 3 was isolated directly from a reaction mixture and the structure shows that, in comparison with the structure of the parent IU(OArP)3, the introduction of a fourth aryloxide results in elongation of the U–P interactions to 3.276 Å to compensate for the steric encumbrance around the uranium centre (see ESI†). The U–O distances, 2.193 Å, are similar to those previously reported for the other complexes featuring this phosphinoaryloxide ligand.13,17 Unfortunately, despite repeated attempts, we have been unable to grow single crystals of 1.NMR spectroscopy
The NMR spectroscopic analysis of the uranium complex 1 shows highly fluxional behaviour, similarly to the parent compound IU(OArP)3 with no resonances being observable in 1H, 29Si, and 31P NMR at ambient temperature. Variable temperature 1H NMR experiments reveal paramagnetically shifted signals at elevated temperatures (see ESI†). In contrast, the homoleptic uranium compound 3 exhibits sharp, paramagnetically-shifted resonances in 1H NMR spectrum in the range 1.38 to 11.73 ppm (see Fig. S7†). No 31P NMR signal is observed again here suggesting that the uranium–phosphine interaction is retained in solution and giving rise to a resonance which is too shifted and/or broadened to observe. The spectroscopic data for the diamagnetic CeIV complex 2 shows fluxional behaviour as 1H NMR resonances for the aromatic protons and the tert-butyl groups are strongly broadened at R.T. and sharper when measured at elevated temperatures (see Fig. S8†). In agreement, the 31P NMR spectrum contains three broadened and overlapping shifts at 23.6, 16.4, and 12.2, which coalesce to a single resonance at 16.23 at elevated temperatures (see Fig. S9†). The trimethylsiloxide 29Si NMR chemical shift is at 6.1 ppm.Lactide polymerisation
Initial studies on the ROP of L-lactide indicate that both 1 and 2 are active catalysts (Fig. 1 and Table S1†). These screening reactions were run under “living” conditions, in the absence of any added BnOH, initiating from the bulky siloxide ligand. Molecular weights obtained from GPC are in good agreement to the theoretical molecular weights, evidenced by monodisperse dispersities (Đ). Less control is observed with 2 (entry 10, Table S1†) compared to the UIV complex 1 (entry 1, Table S1†), as shown by broader Đs. While control is maintained for 1 in toluene, benzene and dichloroethane (DCE), polymerisation in DCE with 2 gives lower conversions and a loss of control (entry 12, Table S1†). The harder Ce(IV) ion in the active species derived from 2 may permit solvent interference or catalyst decomposition. The use of the homoleptic 3 confirms the importance of the siloxide ligand as a reactive initiator. Insertion into the aryloxide of the ligand is sluggish (entry 6, Table S1†), as polymerisation is slow and poorly controlled, and forms polymers with molecular weights twice that expected from conversion.The addition of Ni0 to 1 to form a U–Ni metal–metal bond dramatically alters the polymerisation behaviour. When pre-formed heterobimetallic complex 4 is tested under living or immortal conditions, essentially no polymerisation activity is observed (entries 8 and 9, Table S1†). Our previously reported experimental and computational analyses of 4 show a particularly short, but relatively weak U–Ni bond.13
It may be possible to ascribe this ‘switching off’ by trans-Ni coordination to the Inverse Trans Influence (ITI), the mutual strengthening of two trans-coordinated ligands that occurs in f-block complexes, and is opposite to the weakening effect for the d-block. It is receiving increasing attention in f-block bonding because of its effect on uranyl [UO2]2+ behaviour, and is now attributed primarily to the ligands interacting with pseudocore-like 6p orbitals and inducing a quadrupolar polarisation of the metal core electrons.18 Thus, here the trans-bound Ni strengthens the U–OSiMe3 bond sufficiently to prevent protonolysis or esterification. We should also note that the Ni–P bond in 4 is stronger than the parent U–P bonding in 1. Even though the formal coordination number for UIV is lower than in 1, the loss of hemilability in the OArP ligands upon formation of 4 may hamper monomer access in the subsequent reactivity studies. Calculations of the percent buried volume for the set of UIV complexes UI(OArP)3A and IU(OArP)3Ni (the iodide analogue of 4), and Me3SiOU(OArP)3 (a model made by replacement of CeIV by UIV in the X-ray structure of 2) show almost no difference in accessible space at the initiating ligand (Table S3†).19
Polymerisations of 1 and 2 were also investigated under immortal conditions with a monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst
catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH ratio of 200
BnOH ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
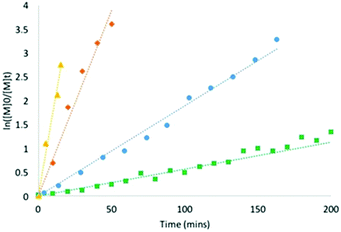
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, where the excess of BnOH as a chain transfer agent permits a decrease in catalyst loading. While both catalysts maintain the exceptional polymerisation control under immortal conditions (entries 5 and 14, Table S1†), remarkable differences were observed in reaction rates. Kinetic studies of both catalyst 1 and 2 were conducted by monitoring the reaction in situ with 1H NMR spectroscopy (Fig. 3 and ESI†). In the absence of BnOH, polymerisation of L-lactide reaches completion in 160 and 600 min for 1 and 2 respectively. The relatively higher affinity of the P donor for U will result in a fixed coordination geometry, with monomer coordination to the productive siloxide face assured. Conversely, a more labile Ce–P bond creates a more open and flexible coordination geometry around 2, enabling non-productive monomer coordination (i.e. trans to the growing chain after phosphine dissociation). However, both U and Ce catalysts are exceptionally faster under immortal conditions: polymerisations are complete in 50 and 15 minutes for 1 and 2, respectively. Intriguingly, the reactivity switches, with the Ce complex now faster. This suggests that the added BnOH may participate in chain exchange reactions with both siloxide and ligand phenoxide groups, alleviating steric congestion and easing monomer access to the more Lewis acidic CeIV centre.
5, where the excess of BnOH as a chain transfer agent permits a decrease in catalyst loading. While both catalysts maintain the exceptional polymerisation control under immortal conditions (entries 5 and 14, Table S1†), remarkable differences were observed in reaction rates. Kinetic studies of both catalyst 1 and 2 were conducted by monitoring the reaction in situ with 1H NMR spectroscopy (Fig. 3 and ESI†). In the absence of BnOH, polymerisation of L-lactide reaches completion in 160 and 600 min for 1 and 2 respectively. The relatively higher affinity of the P donor for U will result in a fixed coordination geometry, with monomer coordination to the productive siloxide face assured. Conversely, a more labile Ce–P bond creates a more open and flexible coordination geometry around 2, enabling non-productive monomer coordination (i.e. trans to the growing chain after phosphine dissociation). However, both U and Ce catalysts are exceptionally faster under immortal conditions: polymerisations are complete in 50 and 15 minutes for 1 and 2, respectively. Intriguingly, the reactivity switches, with the Ce complex now faster. This suggests that the added BnOH may participate in chain exchange reactions with both siloxide and ligand phenoxide groups, alleviating steric congestion and easing monomer access to the more Lewis acidic CeIV centre.
Indeed, 31P NMR spectroscopy supports this idea of BnOH-promoted ligand displacement, as evidenced by growth of a 2-tert-butyl-4-methyl-6-(diphenylphosphino)phenol (HOArP) resonance upon addition of 5 equivalents of BnOH to 2 (Fig. S2†). Of course, siloxide protonolysis will be preferential and 1H NMR spectroscopy supports replacement of OSiMe3 with OBn (Fig. S3†). This ligand displacement even extends to the homoleptic complex 3 where immortal conditions improve control and polymerisation rates (entry 7, Table S1†), suggesting chain exchange creates the new active complex. Short polymer chains of L-lactide using 2 under immortal conditions in a 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio were prepared for end-group analysis. 1H and 2D (COSY, HSQC, HMBC) NMR spectroscopy revealed the resulting PLA chain was capped with an –OH group at one end and PhCH2O– group at the other end (Fig. S4†). Moreover, MALDI mass spectrometry confirms BnOH end group incorporation (Fig. S5†). This is indicative of a coordination insertion mechanism.
5 molar ratio were prepared for end-group analysis. 1H and 2D (COSY, HSQC, HMBC) NMR spectroscopy revealed the resulting PLA chain was capped with an –OH group at one end and PhCH2O– group at the other end (Fig. S4†). Moreover, MALDI mass spectrometry confirms BnOH end group incorporation (Fig. S5†). This is indicative of a coordination insertion mechanism.
Finally, to understand the influence these catalysts have on the stereoselectivity of polymerisations, the ROP of 1 and 2 were examined using rac-lactide; the results are summarised in Fig. 4.
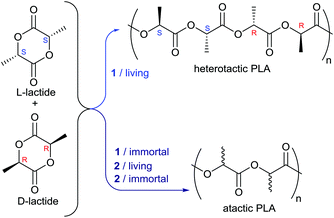 | ||
| Fig. 4 Summary of the differences in reactivity and product tacticity between the UIV and CeIV initiators 1 and 2 under different polymerisation conditions. | ||
Initiator 1 promotes the formation of heterotactic PLA, Pr = 0.79 (entry 2, Table S2†), whereas 2 displays no stereocontrol (entries 8–13, Table S2†). The more rigid coordination environment enforced by the strong U–P bonding induces chain end control stereoselectivity. The more labile bonding, and thus flexible coordination sphere, in Ce reduces any chain end influence, forming atactic PLA. Under immortal conditions, where we believe the coordination geometry opens up in both catalysts, a loss in heterotacticity is observed (Pr = 0.58, entry 7, Table S2†). Beyond chain exchange, reaction conditions also influence tacticity control. The best results are observed in dichloroethane (entry 2, Table S2†), likely due to the higher solubility of rac-lactide. Heterotacticity is also reduced at higher temperatures, with Pr = 0.62 at 90 °C (entry 4, Table S2†). It is noteworthy that, to our knowledge, 1 offers the greatest control over stereoselectivity for any U catalyst for lactide ROP.
While public opinion will likely not let industry make polymers from depleted uranium, the sharply contrasting behaviours of Ce and U, which have almost identical ionic radii, highlight the fundamental differences in 4f- vs. 5f- metal–ligand bonding, an area which is still poorly understood. We have demonstrated both uranium catalyst 1 and cerium catalyst 2 are active initiators in the ROP of lactide. The homoleptic uranium analogue 3 highlights the importance of the siloxide group for a controlled polymerisation and the addition of Ni(0) that forms a U–Ni bond trans to the U–OSiMe3 initiating group in 4 switches off the polymerisation. This could be attributable to the inverse trans influence which would strengthen the U–OSiR3 bond. Model volume calculations suggest the space available at the initiating site is barely changed, but the rigidity of the bound ligands is increased, which could reduce monomer access. However, in the absence of solid-state structures that would enable a detailed comparison of the two initiators, further evidence, such as computational bonding analyses would be required to unequivocally conclude this. We have further shown that under immortal conditions the rates of polymerisation using both 1 and 2 can be substantially increased while maintaining control over the dispersity. Finally, highly heterotactic PLA can be formed using catalyst 1 under living ROP of rac-lactide. Thus, through careful catalyst design and comparison we have made ROP catalysts capable of undergoing living and immortal polymerisations where reactivity can be shut down and tacticity can be switched off through manipulation off the catalyst coordination sphere.
Conflict of interest
There are no conflicts to declare.Acknowledgements
JAH thanks the Austrian Science Fund (FWF) for funding via Erwin Schrödinger Fellowship J3467. FS thanks the University of Edinburgh for funding through the Principal's Career Development Scholarship and the Marie-Curie Actions Programme (FP7-PEOPLE-2013-CIG-618372). PLA and JALW thank the UK EPSRC for funding through grants EP/N022122/1 and EP/M010554/1. We also thank a reviewer for helpful comments on the crystallography.Notes and references
- S. Inkinen, M. Hakkarainen, A.-C. Albertsson and A. Södergård, Biomacromolecules, 2011, 12, 523–532 CrossRef CAS PubMed.
- C. K. Williams and M. A. Hillmyer, Polym. Rev., 2008, 48, 1–10 CrossRef CAS.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- T. R. Forder, M. F. Mahon, M. G. Davidson, T. Woodman and M. D. Jones, Dalton Trans., 2014, 43, 12095–12099 RSC.
- A. J. Chmura, M. G. Davidson, C. J. Frankis, M. D. Jones and M. D. Lunn, Chem. Commun., 2008, 1293–1295 RSC.
- P. L. Arnold, J.-C. Buffet, R. Blaudeck, S. Sujecki, A. J. Blake and C. Wilson, Angew. Chem., Int. Ed., 2008, 47, 6033–6036 CrossRef CAS PubMed.
- P. L. Arnold and Z. R. Turner, Nat. Rev. Chem., 2017, 1, 0002 CrossRef.
- P. L. Arnold, J.-C. Buffet, R. Blaudeck, S. Sujecki and C. Wilson, Chem. – Eur. J., 2009, 15, 8241–8250 CrossRef CAS PubMed.
- C. Bakewell, A. J. P. White, N. J. Long and C. K. Williams, Angew. Chem., Int. Ed., 2014, 126, 9380–9384 CrossRef.
- A. Walshe, J. Fang, L. Maron and R. J. Baker, Inorg. Chem., 2013, 52, 9077–9086 CrossRef CAS PubMed.
- J.-C. Buffet, J. Okuda and P. L. Arnold, Inorg. Chem., 2010, 49, 419–426 CrossRef CAS PubMed.
- E. M. Broderick, N. Guo, T. Wu, C. S. Vogel, C. Xu, J. Sutter, J. T. Miller, K. Meyer, T. Cantat and P. L. Diaconescu, Chem. Commun., 2011, 47, 9897–9899 RSC.
- J. A. Hlina, J. R. Pankhurst, N. Kaltsoyannis and P. L. Arnold, J. Am. Chem. Soc., 2016, 138, 3333–3345 CrossRef CAS PubMed.
- R. Anwander, J. Eppinger, I. Nagl, W. Scherer, M. Tafipolsky and P. Sirsch, Inorg. Chem., 2000, 39, 4713–4720 CrossRef CAS PubMed.
- P. L. Arnold, Z. R. Turner, N. Kaltsoyannis, P. Pelekanaki, R. M. Bellabarba and R. P. Tooze, Chem. – Eur. J., 2010, 16, 9623–9629 CrossRef CAS PubMed.
- M. D. Fryzuk and T. S. Haddad, Chem. Commun., 1990, 1088–1090 RSC.
- J. A. Hlina, J. A. L. Wells, J. R. Pankhurst, J. B. Love and P. L. Arnold, Dalton Trans., 2017, 46, 5540–5545 RSC.
- H. S. La Pierre and K. Meyer, Inorg. Chem., 2013, 52, 529–539 CrossRef CAS PubMed.
- L. Falivene, R. Credendino, A. Poater, A. Petta, L. Serra, R. Oliva, V. Scarano and L. Cavallo, Organometallics, 2016, 35, 2286–2293 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Full details of synthesis, characterisation and lactide polymerisation studies. CCDC 1543395 and 1543396. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt02167d |
| ‡ Current address: Institute of Inorganic Chemistry, Graz University of Technology, Stremayrgasse 9, 8010 Graz, Austria. |
| This journal is © The Royal Society of Chemistry 2017 |