Cationic PCP iridaepoxide and carbene complexes for facile water elimination and activation processes†
Abstract
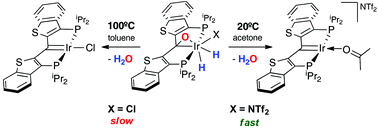
Iridaepoxide dihydride complexes of a PCP ligand bearing benzo[b]thiophene linkers are synthesized through ligand coopertive N2O and H2 activations. These neutral complexes also eliminate water at elevated temperatures to form the corresponding PCcarbeneP complexes which results in the formal hydrogenation of N2O to water. The synthesis of cationic iridaepoxide dihydride complexes are reported herein where the room temperature elimination of water is observed when a donating solvent is used. This supports a previously proposed mechanism for this water elimination where hydrides cis to the epoxide are required. Ir(I) and Ir(III) cationic PCcarbeneP complexes are also synthesized through protonation and through O–H oxidation additions of water and phenol.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...