Open Access Article
Open Access ArticleA structural study of alkaline earth metal complexes with hybrid disila-crown ethers†
Fabian
Dankert
,
Kirsten
Reuter
,
Carsten
Donsbach
and
Carsten
von Hänisch
*
Fachbereich Chemie and Wissenschaftliches Zentrum für Materialwissenschaften (WZMW), Philipps-Universität Marburg, Hans-Meerwein-Straße 4, D-35032, Marburg, Germany. E-mail: haenisch@chemie.uni-marburg.de
First published on 30th November 2016
Abstract
Compounds consisting of [M(1,2-disila-[3n]crown-n)]2+ (M = Mg, Ca, Sr, Ba; n = 5, 6) and [Ba(1,2-disila-benzo[18]crown-6)]2+ cations and different anions were obtained by equimolar reaction of the hybrid disila-crown ethers 1,2-disila[15]crown-5 (1), 1,2-disila[18]crown-6 (2) and 1,2-disila-benzo[18]crown-6 (7) with alkaline earth metal salts. Even with strongly coordinating anions such as Br− or I− stable complexes could be obtained, showing the good coordination ability of these ligands. The structures of all coordination compounds were determined via single crystal X-ray diffraction (XRD). By means of DFT calculations, the complexation ability of 1,2-disila[15]crown-5 (1) towards magnesium bromide was determined to be considerably higher compared to [15]crown-5. The opposite case was observed in solution as the exchange of calcium cations between [18]crown-6 and 1,2-disila[18]crown-6 (2) was studied via dynamic proton nuclear magnetic resonance (NMR) spectroscopy.
Introduction
Within the scope of developing macrocycles, there has also been an increased interest in ring compounds which are made of an inorganic skeleton.1–3 Unlike crown ether complexes, coordination compounds with cyclosiloxanes are very rare. They are mostly constituted of a weakly coordinating anion such as AlF (AlF = [Al{OC(CF3)3}4]−), [SbF6]− or [InH{CH2C(CH3)}3]− as counterion where the charge is widely spread over a non-nucleophilic, chemically robust moiety.2,4–6 The Zr(IV) compound [Zr(D6)Br2][Zr2Br9]2 (D = Me2SiO) is a unique example of a cyclosiloxane complex with a more strongly coordinating halide anion.7One model to describe the reduced basicity of siloxanes is the negative hyperconjugation. An occupied p-orbital of the oxygen atom donates electron density into the σ*-orbital of the silicon–methyl bond, which strengthens the Si–O bond. The complexation of metal ions leads to a competing polarization.2,8 According to Weinhold and West, negative hyperconjugation plays a major role in permethylated siloxanes, which was recently shown via natural-bond-orbital-analysis.9,10 Another approach to explain the low basicity of siloxanes consists of an ionic consideration of the Si–O bond.11–13 Despite the high anionicity of the O atoms, siloxanes do not exhibit an increased coordination ability owing to the energetically unfavoured further polarisation of the already polar Si–O bond. In this respect changes in the nO → σ*Si–C interaction play a significant role.8 Recent work suggests that repulsion between the positively polarized Si atom and the metal ion is eminently important beside the negative hyperconjugation to explain the low coordination ability of cyclosiloxanes.14 Furthermore, extraction experiments have shown that the complexation abilities of ring contracted crown ethers like [17]crown-6 and sila[17]crown-6 are notably smaller than those of common crown ethers revealing that not only electronic effects but also the conformation of the macrocycle determines the complexation ability of such compounds.15,16
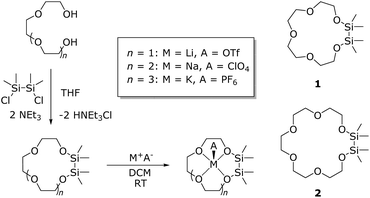
These facts motivated us to incorporate a Si2Me4 unit rather than one SiMe2 in between the oxygen atoms of crown ethers (Scheme 1). Less attention has been paid to this function and its coordination chemistry even though it is known for quite some time.17,18 However, in our recent work on hybrid disila-crown ethers we were able to synthesize crown ethers of the type 1,2-disila[3n]crown-n and their alkali metal complexes. The reaction of 1,2-dichloro-1,1,2,2-tetramethyldisilane and the appropriate glycole yielded the hybrid crown ether. Complexation with selected alkali metal salts in dichloromethane (DCM) then yielded their alkali metal complexes (Scheme 2). By means of DFT calculations as well as dynamic proton NMR experiments, we revealed a comparable complexation ability of these crown ethers and their organic analogues.19 We therefore assume that the reduced complexation ability of siloxanes is rather the result of structural and electrostatic factors. As the next step in understanding the coordination chemistry of disila-crown ethers we herein report the incorporation of alkaline earth metal cations.
Results and discussion
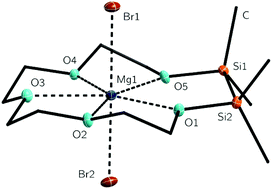
Treatment of 1 with MgBr2 in trifluorotoluene led to the coordination compound [Mg(1,2-disila[15]crown-5)Br2] (3). Neat 3 is a white powder which can be recrystallized after dissolution in DCM and layering with n-pentane. The resulting colourless plates were analysed via XRD. 3 crystallizes in the orthorhombic space group Pbca. The magnesium cation is coordinated by all oxygen atoms of the cyclic ligand as well as by two bromide ions giving a coordination number of seven (Fig. 1). The oxygen atoms are in almost coplanar arrangement with the magnesium cation, which is apparent from the Br1–Mg1–O–angles of approximately 90°. The MgBr2 fragment is in well-nigh linear shape showing a Br1–Mg1–Br2 angle of 178.5(1)°. This is consistent with the [Mg(THF)4Br2] (THF = tetrahydrofuran) complex, whose Br–Mg–Br-angle is 178.0(1)°.20The O–Mg–O-angles differ from 67.5(1) to 81.5(1)°. The O1–Mg1–O5-angle is enlarged as a result of the Si–Si bond of 232.2(1) pm. The methyl groups are taking an almost ecliptic arrangement, which can be seen in the C–Si–Si–C-torsion angles of 9.3(1)° and 6.2(1)°. This eclipsed arrangement was also reported for different cyclosiloxane and disila-crown ether complexes.2,4,14,19,21 The O–Mg-bond lengths vary from 223.3(2) to 235.1(2) pm. Hence they are slightly longer than the O–Mg-bond lengths in related compounds with [15]crown-5 as ligand (see Table 1) and quite as long as those of [Mg([18]crown-6)(Cl–H–Cl)2].22 However, the O–Mg-bond lengths of fully carbon substituted oxygen atoms in 3 are comparable to those which are half carbon and half silicon substituted, so there is no hint for lower complexation ability of the silicon bonded oxygen atoms.
| Compound | Ocrown–M [pm] | CNa | Ref. |
|---|---|---|---|
| a Coordination number. b This work. c All symmetry generated. | |||
| 3 | 223.3(2)–235.1(2) | 7 | |
| [Mg([15]crown-5)(SCPh3)2] | 214.3(3)–219.2(4) | 7 | 26 |
| [Mg([15]crown-5)(H2O)2](NO3)2 | 201.2(7)–235.7(8) | 7 | 27 |
| [Mg([15]crown-5)(NCS)2] | 214.2(2)–223.9(7) | 7 | 28 |
| [Mg([15]crown-5)([CuCl4])CH3CN] | 211.7(4)–219.1(3) | 7 | 29 |
| 4 | 243.6(1)–247.9(1) | 7 | |
| [Ca([18]crown-6)(OTf)2(H2O)] | 255.5(1)–265.7(1) | 9 | 30 |
| [Ca([18]crown-6)I2] | 264.9(5)c | 8 | 31 |
| [Ca([15]crown-5)(NCS)2H2O] | 251.2(2)–258.5(2) | 8 | 28 |
| [Ca([15]crown-5)(NO3)2] | 247.4(3)–258.1(4) | 9 | 27 |
| 5 | 270.0(2)–277.7(2) | 8 | |
| [Sr([18]crown-6)(NO3)2] | 266.7(5)–275.5(3) | 10 | 27 |
| [Sr([18]crown-6)(HSO4)2] | 268.2(8)–268.0(8) | 10 | 32 |
| [Sr([18]crown-6)(H2O)3][CuCl4] | 267.2(3)–270.0(3) | 9 | 33 |
| [Sr([18]crown-6)(MeCN)3][BPh4]2 | 262.4(2)–271.4(2) | 9 | 34 |
| [Sr([18]crown-6)(hmpa)2][Sn(SnPh3)3]2 | 258.9(4)–273.6(4) | 8 | 35 |
The shortest O–Mg bond is attributed to the silicon bonded oxygen atom O5 whereas the longest O–Mg bond is observed for the fully carbon substituted oxygen atom O3. The relative binding affinity of the hybrid ligand 1 towards magnesium bromide was further studied by quantum chemical calculations and is presented in Scheme 3. The exchange of magnesium bromide from [15]crown-5 to 1,2-disila[15]crown-5 (1) was calculated by means of DFT using the BP86 functional and def2-TZVP basis sets with inclusion of dispersion interactions together with charge compensation and is energetically favoured by 217.63 kJ mol−1. This result implies a significantly better coordination ability of 1 compared to [15]crown-5 and is in well accordance to previous calculations for 1,2-disila[12]crown-4 towards Li+.19 Unfortunately, the results of quantum chemical calculations could not be underlined with experimental data such as dynamic 1H NMR spectroscopy. 3 does only barely dissolve in DCM and thus no proton NMR study was possible for this compound.
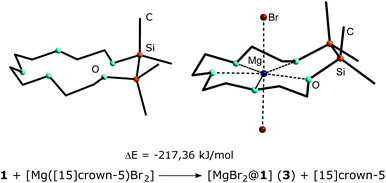 | ||
| Scheme 3 Optimized structures of 1 (left) and 3 (right) and relative energies of the cation exchange from [15]crown-5 to 1,2-disila[15]crown-5 (1). Calculations performed at the def2-TZVP level of theory. For XYZ-Data and related structures see ESI.† | ||
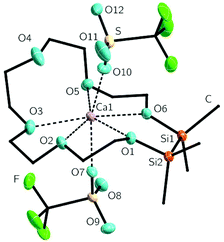
The heavier calcium cation was incorporated by treating 2 with Ca(OTf)2 (OTf = F3CSO3−) in DCM. The resulting coordination compound [Ca(1,2-disila[18]crown-6)OTf2] (4) was obtained as colourless powder. After recrystallization from dichloromethane, single crystals suitable for XRD were obtained in shape of colourless rods. 4 crystallizes in the monoclinic space group P21/n. This coordination compound shows a mismatch: one of the crown ether oxygen atoms does not participate in the coordination of the central atom. The crown acts as “pseudo-1,2-disila[15]crown-5”, so overall five crown ether oxygen atoms together with the two triflate groups coordinate to the calcium cation giving a total coordination number of seven (Fig. 2). The O4–Ca1 atomic distance is 321.4(1) pm. The distorted O7–Ca1–O10 bond angle of 163.0(1)° is a result of the repulsion between O4 and one of the triflate groups. Hence this angle is slightly smaller than the expected 180° angle which is found in [Ca(CH3CH2OH)4OTf2].23
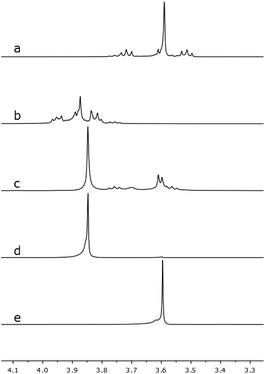
The O7–Ca1–O(1,2,3,5,6) bond angles differ from 78.3(1) to 99.41(1)° leading to a distorted arrangement of the crown ether oxygen atoms around the calcium cation. The mismatch is obviously caused by the small ion diameter of Ca2+, which is 200 pm, and the enlarged cavity of the ligand 2 compared to [18]crown-6.24 The coordination pattern of 4 is known for different crown ether complexes.22,25 However, no mismatched calcium complexes of [18]crown-6 are known to date. Due to the mismatch, the Ocrown–Ca bond lengths in 4 are slightly shorter than those of complexes of [18]crown-6 and close to [15]crown-5 complexes of calcium (see Table 1). In case of this mismatched crown ether complex, we observed a significantly lower complexation ability of 2 in comparison to [18]crown-6 which was confirmed by means of dynamic 1H NMR spectroscopy (see Scheme 4). An equimolar reaction of 2, [18]crown-6 and Ca(OTf)2 was carried out. The percentage of coordinated and free ligand was calculated from the average chemical shifts in the mixture according to36
| δaverage = (ncoordinatedδcoordinated + nnoncoordinatedδnoncoordinated)/ntotal |
Chemical shifts of compound 4 are significantly high-field shifted in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4 and [18]crown-6, whereas the singlet of [18]crown-6 is significantly down-field shifted (see Scheme 4, spectrum c). As calculated with the above given equation, the ratio of 4 to [Ca([18]crown-6)OTf2] is 14
1 mixture of 4 and [18]crown-6, whereas the singlet of [18]crown-6 is significantly down-field shifted (see Scheme 4, spectrum c). As calculated with the above given equation, the ratio of 4 to [Ca([18]crown-6)OTf2] is 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86. This result is contradicted to experimental data19 which was observed for the system 1,2,7,8-tetrasila[12]crown-4/LiPF6/[12]crown-4 but attention should be paid to the enlarged cavity size of 2.
86. This result is contradicted to experimental data19 which was observed for the system 1,2,7,8-tetrasila[12]crown-4/LiPF6/[12]crown-4 but attention should be paid to the enlarged cavity size of 2.
As reported for different supramolecules, the coordination ability of such compounds highly depends on the characteristics of the host-molecule. Especially the size complementarity between cation and host cavity influences the magnitude of the binding constant.37 The molecular structure of [Ca([18]crown-6)(OTf)2(H2O)] reveals that [18]crown-6 enables all six oxygen atoms for the coordination to the metal ion rather than only five as in 4.30 This is presumably the key advantage of [18]crown-6 over the corresponding disila crown ether 2.
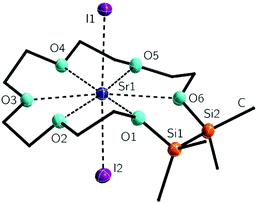
The halide complex [Sr(1,2-disila[18]crown-6)I2] (5) was synthesized by reaction of 2 with SrI2 in trifluorotoluene. After recrystallization, 5 was obtained as colourless blocks which were suitable for XRD. 5 crystallizes in the monoclinic space group C2/c with one solvent molecule DCM. In contrast to 4, all crown ether oxygen atoms are coordinating to the larger strontium cation (Fig. 3). With two additional iodine atoms, the strontium cation has a coordination number of eight. No crown ether complex of the halide salt SrI2 is known to date. Actually, different approaches to obtain crown ether complexes of strontium halides yielded [Sr([12]crown-4)(H2O)3Br]Br or [Sr([18]crown-6)(H2O)3]X2 (X = Cl−, Br−). Therein no or no more than one halide anion is placed in the coordination sphere of the metal ion.38,39 Having an ion diameter of 236 pm, the strontium cation is still too small for the hole diameter of 2.24 The twisted arrangement of the ligand enables all oxygen atoms to participate in the coordination. The O–Sr1–I1 bond angles differ from 86.1(1) to 96.9(1)° stronger than for example the O–Mg1–Br1 bond angles in 3. In addition, the Sr(OSiMe2)2 fragment shows an envelope conformation where the disilane-unit is buckling with Sr–O–O–Si torsion angles of 151.0(1) and 146.8(1)°. The methyl groups are again in an almost eclipsed arrangement with C–Si–Si–C torsion angles of 5.1(1) and 6.2(1)°. The I1–Sr1–I2 bond angle of 177.4(1)° is very close to 180° showing a well-nigh linear coordination of the SrI2-fragment, which is also observed for [Sr(DME)2(THF)I2] (DME = 1,2-dimethoxyethane), whose I–Sr–I bond angle is 178.6(1)°.40
With bond lengths of 320.1(1) and 320.5(1) pm, the Sr–I bonds are similar to those of different ether adducts such as for example [Sr(DME)3I2], [Sr(diglyme)2I2] (diglyme = bis(2-methoxyethyl)ether) or [Sr(DME)2(THF)I2].40 Again all carbon substituted oxygen atoms show almost the same bond lengths to the metal ion as the half carbon and half silicon affected oxygen atoms (270.0(2) to 277.7(2) pm). However, the O6–Sr1 bond is the longest one, but should be considered with respect to the twisting of the crown ether. The Sr–Ocrown bonds of [Sr([18]crown-6)(NO3)3] and [Sr([18]crown-6)(HSO4)2] are marginally shorter.27,32 The cationic crown compounds [Sr([18]crown-6)(hmpa)2]2+ (hmpa = hexamethylphosphoramide) and [Sr([18]crown-6)L3]2+ (L = H2O, CH3CN) show notably smaller Sr–Ocrown bond lengths (see also Table 1).33,34,39
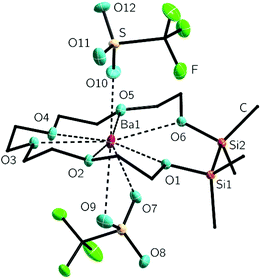
Finally, the heaviest non-radioactive alkaline earth metal was incorporated into the macrocycle by treating 2 with Ba(OTf)2 in DCM. After recrystallization, [Ba(1,2-disila[18]crown-6)OTf2] (6) crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with two independent molecules within the asymmetric unit. The metal ion is coordinated by all of the crown ether oxygen atoms as well as by the two triflate groups. One triflate group is coordinating as bidentate ligand giving the barium cation a total coordination number of nine (Fig. 4). With its ion diameter of 270 pm the barium cation is larger than the strontium cation, but the crown is still twisting as shown by the O5–Ba1–O10 and O6–Ba1–O10 bond angles, which are 78.1(1) and 77.4(1)° respectively.24 The O1–4–Ba1–O10 angles are much closer to 90°. The Ocrown–Ba bond lengths of 277.5(1) to 285.9(1) pm are in accordance with those of different [18]crown-6 complexes with barium. Nevertheless these distances are dependent on several factors such as coordination number, donor strength and the sterically demand of coligands (see Table 2).
with two independent molecules within the asymmetric unit. The metal ion is coordinated by all of the crown ether oxygen atoms as well as by the two triflate groups. One triflate group is coordinating as bidentate ligand giving the barium cation a total coordination number of nine (Fig. 4). With its ion diameter of 270 pm the barium cation is larger than the strontium cation, but the crown is still twisting as shown by the O5–Ba1–O10 and O6–Ba1–O10 bond angles, which are 78.1(1) and 77.4(1)° respectively.24 The O1–4–Ba1–O10 angles are much closer to 90°. The Ocrown–Ba bond lengths of 277.5(1) to 285.9(1) pm are in accordance with those of different [18]crown-6 complexes with barium. Nevertheless these distances are dependent on several factors such as coordination number, donor strength and the sterically demand of coligands (see Table 2).
| Compound | Ocrown–Ba [pm] | CNa | Ref. |
|---|---|---|---|
| a Coordination number. b Mes* = 2,4,6-tBu3C6H2. c This work. d pta = 1,1,1-trifluoro-5,5-dimethylhexane-2,4-dione. e DB = dibenzo. | |||
| [Ba([18]crown-6)(hmpa)2][BPh4]2 | 277.2(2)–281.3(2) | 8 | 34 |
| [Ba([18]crown-6)(hmpa)2][SnPh3] | 277(1)–280(1) | 8 | 35 |
| [Ba([18]crown-6)(HCPh2)2] | 275.0(2)–280.2(3) | 8 | 41 |
| [Ba([18]crown-6)(PPh)2] | 271.7(2)–280.7(2) | 8 | 42 |
[Ba([18]crown-6)(hmpa)2](SeMes*)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
276.7(3)–280.3(2) | 8 | 43 |
| 6 | 277.5(1)–285.9(1) | 9 | |
| 8 | 277.5(2)–289.9(2) | 9 | |
| [Ba([18]crown-6)(NCS)2(H2O)] | 280.8(6)–287.5(5) | 9 | 44 |
| [Ba([18]crown-6){O2P(OnBu)2}(H2O)] | 281(2)–290(2) | 9 | 45 |
| [Ba([18]crown-6)(O2CCF3)2(py)] | 282.0(5)–284.5(7) | 10 | 46 |
| [Ba([18]crown-6)(pta)2]d | 278.6(3)–283.1(3) | 10 | 47 |
| [Ba(DB[18]crown-6)(pta)2]e | 292.9(4)–317.1(4) | 10 | 47 |
| [Ba([18]crown-6)(H2O)3Cl]Cl | 277.0(2)–285.4(1) | 10 | 48 |
| [Ba([18]crown-6)(O2CCH3)2] | 275.3(2)–283.5(2) | 10 | 49 |
| [Ba([18]crown-6)(NO3)2H2O] | 283(1)–290.8(8) | 11 | 27 |
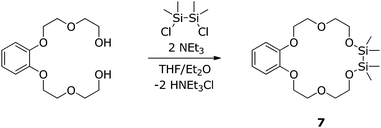
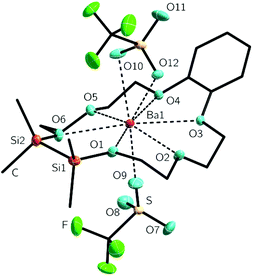
Similar to the compounds 3–5, the silicon bonded oxygen atoms do not differ significantly concerning the oxygen metal distance in comparison to the fully carbon substituted oxygen atoms. Again an eclipsed arrangement of the methyl groups at the silicon atoms is found. With 2.3(1) and 2.7(1)° the C–Si–Si–C torsion angles are even smaller. The monodentate coordinating triflate group binding to the metal ion has a O(10)–Ba1 bond length of 253.9(3) pm. The chelating triflate group has significantly longer bond lengths of 288.7(1) and 292.1(1) pm. This is very similar to the trifluoroacetate groups in the complex [Ba(O2CCF3)2([18]crown-6)(py)] (py = pyridine). The trifluoroacetate group, which acts as monodentate ligand, has O–Ba bond length of 263.1(9) pm. The chelating one has a bond length of 287.6(1) pm.46 To what extend these complexes are affected by negative hyperconjugation is still a matter of interest. We therefore claimed to crystallize a free ligand which can be compared to coordination compounds and started to prepare the heavier benzylic crown ether 1,2-disila-benzo[18]crown-6 (7). The organic fragment of 1,2-disila-benzo[18]crown-6 was synthesized by reaction of catechol and 2-(2-chlorethoxy)ethanol.50 Subsequent addition of 1,2-dichloro-1,1,2,2-tetramethyldisilane yielded compound 7 (Scheme 5). However, 7 was obtained as a viscous oil, so no crystal structure could be obtained. An investigation regarding the coordination ability of 7 was still interesting because the benzylic unit as well as the Si2Me4 fragment are considerably unpliable. Coordination chemistry was performed by reaction of 7 with Ba(OTf)2, yielding [Ba(1,2-disila-Benzo[18]crown-6)OTf2] (8), which crystallizes in the orthorhombic space group P212121 (Fig. 5).
As previously shown for 6, the metal ion in 8 is coordinated by all of the crown ether oxygen atoms as well as the two triflate groups. The barium ion is only slightly shifted out of the center of the crown ether. The transannular angles of 157.0(1) to 170.0(1)° are close to 180°, so the barium cation fits well into the cavity of 7.
In contrast to 6, the twisting of the crown is absent. The crown ether is rather bent with the benzylic as well as the disilane unit buckling to one side. With 277.5(2)–289.9(2) pm, the Ocrown–Ba distances have almost the same lengths as in 6. So have those of related compounds (see Table 2). Similar to 6, one triflate group is chelating, the other one bonds to the metal ion only by a single oxygen atom. In comparison to O9, whose atomic distance to the metal ion is 263.0(7) pm, the chelating oxygen atoms of the triflate group have O10–Ba1 and O12–Ba1 atomic distances of 282.8(4) and 279.1(6) pm, respectively. The methyl groups of the SiMe2 units are only slightly contorted to each other and show torsion angles of 16.8(1) and 18.3(1)°.
According to recently published work another key role in understanding the complexation ability of silicon based crown ethers analogues is the repulsion between the positively charged metal ion and the positively polarized silicon atoms.14 The use of Si2Me4 rather than one SiMe2 allows lower repulsion, which can be seen in the average Si–M distances. This might also be an explanation for the higher complexation ability of these hybrid disila-crown ethers in comparison to cyclosiloxanes which we reported before. Further calculations would be helpful to quantify though. Experimental data of metal complexes with disila units, however, features longer average Si–M distances than the sila units in siloxanes. While [Li(1,2-disila[12]crown-4)OTf] has average M–Si distances of 321.6 pm they are shortened to 272–275 pm in the cyclosiloxane complexes [Li(D6)AlF] and [Li(D6)AlPhF] (AlPhF = [Al{OC(CF3)2Ph}4]−).2,19 [K(1,2-disila[18]crown-6)PF6] has Si–M average distances of 392.4(1) pm.19 [K(D7)]+ has average distances of 358–365 pm, so these are shortened as well.4,21 Analogous observations were made in the comparison of alkaline earth metal complexes reported in this work like 5 and 6 with siloxane complexes such as [Sr(NBN-SiMe2OSiMe2O)THF]2 or [Ba(THF)6(dmpz)8{(OSiMe2)O}2] (dmpz = 3,5-dimethylpyrazolate).51,52 Still no complexes of common cyclosiloxanes and alkaline earth metal salts are known today, so the reported complexes in this work are the first crown-type structures containing an alkaline earth metal as well as ligands bearing Si–O donors.
Conclusions
In a recent work, we described the synthesis of hybrid disila-crown ethers of the type 1,2-disila[3n]crown-n (1: n = 5, 2: n = 6) and the formation of alkaline metal complexes.19 Within this study, we reported the synthesis of 1,2-disila-benzo[18]crown-6 (7) using similar conditions.By equimolar reaction of alkaline earth metal salts with the disila-crown ethers 1, 2 and 7, we obtained the alkaline earth metal complexes 3–6 and 8. The structures of all coordination compounds were determined via X-ray crystallographic studies. The Ocrown–M bond lengths indicate that all carbon substituted oxygen atoms show almost the same bond lengths to the metal ion as the half carbon and half silicon affected oxygen atoms, so that on a structural level no reduced complexation ability of these O–Si2Me4–O fragments can be observed. The variation of ion diameter and cavity diameter of the crown then gives interesting coordination patterns: in compound 4, the cavity diameter of 2 is too large for the calcium ion resulting in a mismatch structure. This structure reveals that it's not inevitably the silicon affected oxygen atom which does not participate in the coordination but according to proton NMR-studies, the complexation ability of 1,2-disila[18]crown-6 (2) was determined to be significantly lower than those of [18]crown-6. The size complementarity between cation and host cavity significantly influences the complex stabilities.
Beside the triflate complexes 4, 6 and 8, we also obtained complexes with strongly coordinating anions such as Br− or I− (compound 3 and 5). These results show a better coordination ability of disila-bridged donor atoms in comparison to R2Si bridges, which results from longer Si–M distance within the metal complexes and a reduced cyclic stress. Furthermore DFT calculations (BP86 functional and def2-TZVP basis set) revealed that the coordination ability of 1,2-disila[15]crown-5 (1) towards magnesium bromide is considerably higher compared to [15]crown-5. Nonetheless, all disila-substituted crown ether oxygen atoms of the type SiSi–O–SiSi would still be useful to give more meaningful results in how far disila-affected crown ethers can challenge organic crown compounds. Most recent work hints a considerably higher complexation ability of the partially silicon substituted crown ether 1,2,4,5-tetrasila[12]crown-4 towards Li+ than [12]crown-4.53 Thus, further investigations for this kind of crown ethers and a stepwise synthesis of a fully silicon substituted crown ether are intended for the future.
Experimental section
General
All working procedures were carried out with rigorous exclusion of oxygen and moisture using Schlenk techniques under inert gas atmosphere. Solvents were dried and freshly distilled before use. Compounds 1, 2 and the organic fragment of 7 were synthesized using methods described in literature.19,50 Alkaline earth metal salts were stored and handled under argon atmosphere using a glovebox. NMR spectra were recorded on a Bruker AV III HD 300 MHz or AV III 500 MHz. Infrared (IR) spectra of the respective samples were measured using attenuated total reflectance (ATR) mode on a Bruker Model Alpha FT-IR. MS-spectrometry was measured on JEOL AccuTOF-GC (LIFDI) or LTQ-FT (ESI). Elemental analysis was performed on a Vario MicroCube. Fluorine-containing compounds led to ongoing damage of the elemental analyser and were not measured.1H NMR: (300 MHz, CD2Cl2) δ = 0.44 (s, 12H, Si(CH3)2), 3.77–3.80 (m, 4H, CH2), 3.86 (s, 8H, CH2), 3.95–3.98 (m, 4H, CH2) ppm; 13C{1H} NMR: (75 MHz, CD2Cl2) δ = −0.8 (s, Si(CH3)2), 61.8 (s, CH2), 68.1 (s, CH2), 68.5 (s, CH2), 69.9 (s, CH2) ppm; 29Si{1H} NMR: (99 MHz, CD2Cl2) δ = 18.0 (s, Si(CH3)2) ppm; MS: LIFDI(+) m/z (%): 411.051 [M − Br]+ (83); IR (cm−1): 2948 (m), 2884 (w), 1634 (w), 1467 (m), 1345 (w), 1297 (w), 1243 (m), 1084 (s), 1058 (s), 1046 (s), 959 (s), 922 (m), 896 (m), 824 (m), 803 (m), 774 (w), 730 (s), 696 (w), 639 (w), 568 (w).
1H NMR: (300 MHz, CD2Cl2) δ = 0.41 (s, 12H, Si(CH3)2), 3.80–3.84 (m, 4H, CH2), 3.87–3.89 (m, 12H, CH2), 3.94–3.97 (m, 4H, CH2) ppm; 13C{1H} NMR: (75 MHz, CD2Cl2) δ = −0.9 (s, Si(CH3)2), 62.9 (s, CH2), 69.7 (s, CH2), 70.3 (s, CH2), 70.7 (s, CH2), 72.7 (s, CH2), 120.7 (q, 1JCF = 318 Hz, CF3) ppm; 29Si{1H} NMR: (99 MHz, CD2Cl2) δ = 19.7 (s, Si(CH3)2) ppm; 19F NMR: (282 MHz, CD2Cl2) δ = −79.3 (s, CF3) ppm; MS LIFDI(+) m/z (%): 375.165 [1,2-disila[18]crown-6 + Na]+ (100), 541.088 [M − OTf]+ (40); IR (cm−1): 2954 (w), 2887 (w), 1471 (w), 1357 (w), 1305 (s), 1240 (s), 1219 (s), 1164 (s), 1059 (s), 1028 (s), 935 (s), 868 (m), 838 (s), 817 (s), 795 (s), 775 (s), 760 (s), 726 (s), 634 (s), 579 (m), 514 (s), 460 (w).
1H NMR: (300 MHz, CD2Cl2) δ = 0.46 (s, 12H, Si(CH3)2), 3.81–3.84 (m, 4H, CH2), 3.90 (s, 4H, CH2), 3.91–3.96 (m, 8H, CH2), 4.05–4.08 (m, 4H, CH2) ppm; 13C{1H} NMR: (75 MHz, CD2Cl2) δ = 0.0 (s, Si(CH3)2), 63.1 (s, CH2), 70.4 (s, CH2), 70.5 (s, CH2), 70.5 (s, CH2), 73.0 (s, CH2) ppm; 29Si{1H} NMR: (99 MHz, CD2Cl2) δ = 18.2 (s, Si(Me)2) ppm. MS: LIFDI(+) m/z (%): 566.984 [M − I]+ (100); IR (cm−1): 2943 (w), 2881 (w), 1457 (w), 1353 (w), 1320 (w), 1245 (m), 1082 (s), 1039 (s), 972 (s), 957 (s), 930 (s), 919 (s), 862 (s), 841 (s), 823 (s), 797 (s). 769 (s), 729 (s), 696 (m), 657 (w), 628 (m), 532 (w), 461 (w).
1H NMR: (300 MHz, CD2Cl2) δ = 0.36 (s, 12H, Si(CH3)2), 3.68–3.70 (m, 4H, CH2), 3.78 (s, 12H, CH2), 3.86–3.88 (m, 4H, CH2) ppm; 13C{1H} NMR: (75 MHz, CD2Cl2) δ = −0.9 (s, Si(CH3)2), 63.6 (s, CH2), 70.7 (s, CH2), 70.9 (s, CH2), 71.3 (s, CH2), 73.2 (s, CH2), 120.9 (q, 1JCF = 321 Hz, CF3) ppm; 19F NMR: (282 MHz, CD2Cl2) δ = −79.1 (s, CF3) ppm; 29Si{1H} NMR: (99 MHz, CD2Cl2) δ = 17.6 (s, Si(CH3)2) ppm; MS ESI(+) m/z (%): 639.031 [M − OTf]+ (100); IR (cm−1): 2951 (w), 2925 (w), 2888 (w), 1475 (w), 1456 (w), 1353 (w), 1296 (s), 1242 (s), 1223 (s), 1164 (s), 1123 (s), 1105 (s), 1083 (s), 1074 (s), 1024 (s), 964 (s), 952 (s), 917 (w), 896 (w), 873 (m), 864 (s), 842 (s), 821 (s), 799 (m), 774 (s), 759 (m), 736 (s), 719 (m), 698 (w), 631 (s), 580 (s), 574 (s), 540 (s), 514 (s), 456 (w).
1H NMR: (300 MHz, CD3CN) δ = 0.21 (s, 12H, Si(CH3)2), 3.60–3.63 (m, 4H, CH2), 3.75–3.80 (m, 8H, CH2), 4.08–4.11 (m, 4H, CH2), 6.87–6.98 (m, 4H, CHAR) ppm; 13C{1H} NMR: (75 MHz, CD3CN) δ = −0.29 (s, Si(CH3)2), 63.9 (s, CH2), 69.8 (s, CH2), 70.4 (s, CH2), 73.6 (s, CH2), 114.7 (s, CH2), 122.2 (s, CHAr), 149.8 (s, CqAr) ppm. 29Si{1H} NMR: (99 MHz, CD3CN): δ = 11.0 (s, Si(CH3)2) ppm; MS (LIFDI+) m/z (%): 400.17443 [M]+ (20); IR (cm−1): 2947 (m), 2868 (m), 1593 (m), 1501 (s), 1453 (m), 1396 (w), 1356 (w), 1328 (w), 1248 (s), 1219 (s), 1125 (s), 1088 (s), 1051 (s), 943 (s), 826 (s), 789 (s), 763 (s), 740 (s), 682 (w), 658 (m), 630 (w), 481 (w).
1H NMR: (300 MHz, CD2Cl2) δ = 0.36 (s, 12H, Si(CH3)2), 3.81–3.88 (m, 8H, CH2), 4.03–4.06 (m, 4H, CH2), 4.24–4.27 (m, 4H, CH2), 6.93–7.03 (m, 4H, CHAR) ppm; 13C{1H} NMR: (75 MHz, CD2Cl2) δ = −0.9 (s, Si(CH3)2), 63.4 (s, CH2), 68.4 (s, CH2), 69.6 (s, CH2), 73.4 (s, CH2), 112.3 (s, CHAR), 120.8 (q, 1JCF = 319 Hz, CF3), 122.8 (s, CHAr), 146.9 (s, Cq,Ar) ppm; 19F NMR: (282 MHz, CD2Cl2) δ = −78.7 (s, CF3) ppm; 29Si{1H} NMR: (99 MHz, CD2Cl2) δ = 17.7 (s, Si(CH3)2) ppm; MS LIFDI(+) m/z (%): 687.031 [M − OTf]+ (100), 1523.014 [2M − OTf]+ (100); IR (cm−1): 2945 (w), 2885 (w), 1596 (w), 1506 (s), 1477 (m), 1456 (w), 1357 (s), 1300 (s), 1240 (s), 1220 (s), 1171 (s), 1115 (s), 1063 (s), 1034 (s), 1023 (s), 961 (s), 941 (s), 914 (s), 865 (m), 852 (s), 837 (s), 819 (s), 795 (s), 772 (s), 756 (s), 737 (s), 634 (s), 604 (m), 583 (m), 573 (m), 528 (m), 513 (s), 454 (w).
Computational details
Calculations were performed with the program system TURBOMOLE V7.0.1.54 The resolution of identity (RI) approximation, dispersion corrections, and the conductor-like screening model (COSMO) were applied throughout, the latter with default settings. For all calculations, the BP86 functional was chosen, utilizing a def2-TZVP basis set.55Crystal structures
Single crystal X-ray diffraction was carried out using Bruker D8 Quest (3, 6, 8) or IPDS 2 diffractometer (4, 5) at 100–110 K with MoKα radiation and X-ray optics or graphite monochromatization, respectively (λ = 0.71073). The structures were solved by direct methods and refinement with full-matrix-least-squares against F2 using SHELXT- and SHELXL-2015 on OLEX2 platform.56–58 Triflate groups were refined with help of DSR.59 Crystallographic data for compounds 3–6 and 8 are denoted as follows: CCDC No. 1497469 (3), 1497467 (4), 1497468 (5·DCM), 1497471 (6) and 1497470 (8).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 357, independent reflections 3841 [R(int) = 0.0724], 203 parameters, R-index [I ≥ 2σ(I)] 0.0322, wR2 (all data) 0.0553, GOOF 1.023, Δρmax, Δρmin 0.40/−0.41 e Å3.
357, independent reflections 3841 [R(int) = 0.0724], 203 parameters, R-index [I ≥ 2σ(I)] 0.0322, wR2 (all data) 0.0553, GOOF 1.023, Δρmax, Δρmin 0.40/−0.41 e Å3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 664, independent reflections 4991 [R(int) = 0.0780], 358 parameters, R-index [I ≥ 2σ(I)] 0.0408, wR2 (all data) 0.1089, GOOF 1.008, Δρmax, Δρmin 0.73/–0.67 e Å3.
664, independent reflections 4991 [R(int) = 0.0780], 358 parameters, R-index [I ≥ 2σ(I)] 0.0408, wR2 (all data) 0.1089, GOOF 1.008, Δρmax, Δρmin 0.73/–0.67 e Å3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303, independent reflections 5516 [R(int) = 0.0290], 257 parameters, R-index [I ≥ 2σ(I)] 0.0191, wR2 (all data) 0.0360, GOOF 0.800, Δρmax, Δρmin 0.60/−0.61 e Å3.
303, independent reflections 5516 [R(int) = 0.0290], 257 parameters, R-index [I ≥ 2σ(I)] 0.0191, wR2 (all data) 0.0360, GOOF 0.800, Δρmax, Δρmin 0.60/−0.61 e Å3.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 4, Z′ = 2, 100 K, a = 9.7531(4) Å, b = 17.5485(8) Å, c = 18.5666(8) Å, α = 72.5040(10)°, β = 86.902(2)° γ = 89.5490(10)°, V = 3026.2(2) Å3, ρ = 1.617 g cm−3, multiscan absorption correction using SADABS-2014,60μ = 1.617 mm−1, Tmin, Tmax = 0.544, 1.000, 2Θ range 4.518–61.68°, reflections measured 103
, Z = 4, Z′ = 2, 100 K, a = 9.7531(4) Å, b = 17.5485(8) Å, c = 18.5666(8) Å, α = 72.5040(10)°, β = 86.902(2)° γ = 89.5490(10)°, V = 3026.2(2) Å3, ρ = 1.617 g cm−3, multiscan absorption correction using SADABS-2014,60μ = 1.617 mm−1, Tmin, Tmax = 0.544, 1.000, 2Θ range 4.518–61.68°, reflections measured 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763, independent reflections 18
763, independent reflections 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 918 [R(int) = 0.0254], 982 parameters, R-index [I ≥ 2σ(I)] 0.0237, wR2 (all data) 0.0509, GOOF 1.093, Δρmax, Δρmin 1.03/−2.70 e Å3.
918 [R(int) = 0.0254], 982 parameters, R-index [I ≥ 2σ(I)] 0.0237, wR2 (all data) 0.0509, GOOF 1.093, Δρmax, Δρmin 1.03/−2.70 e Å3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 181, independent reflections 8616 [R(int) = 0.0346], 525 parameters, R-index [I ≥ 2σ(I)] 0.0274, wR2 (all data) 0.0481, GOOF 1.037, Δρmax, Δρmin 0.59/−0.60 e Å3, Flack parameter 0.002(6).
181, independent reflections 8616 [R(int) = 0.0346], 525 parameters, R-index [I ≥ 2σ(I)] 0.0274, wR2 (all data) 0.0481, GOOF 1.037, Δρmax, Δρmin 0.59/−0.60 e Å3, Flack parameter 0.002(6).
Acknowledgements
This work was financially supported by the Deutsche Forschungsgemeinschaft (DFG).References
- J. S. Ritch and T. Chivers, Angew. Chem., 2007, 119, 4694–4697 ( Angew. Chem., Int. Ed. , 2007 , 46 , 4610–4613 ) CrossRef.
- A. Decken, J. Passmore and X. Wang, Angew. Chem., 2006, 118, 2839–2843 ( Angew. Chem., Int. Ed. , 2006 , 45 , 2773–2777 ) CrossRef.
- C. von Hänisch, O. Hampe, F. Weigend and S. Stahl, Angew. Chem., 2007, 119, 4859–4863 ( Angew. Chem., Int. Ed. , 2007 , 46 , 4775–4779 ) CrossRef.
- M. R. Churchill, C. H. Lake, S.-H. L. Chao and O. T. Beachley, J. Chem. Soc., Chem. Commun., 1993, 1, 1577–1578 RSC.
- A. Decken, F. A. LeBlanc, J. Passmore and X. Wang, Eur. J. Inorg. Chem., 2006, 4033–4036 CrossRef CAS.
- I. Krossing and I. Raabe, Angew. Chem., 2004, 116, 2116–2142 ( Angew. Chem., Int. Ed. , 2004 , 43 , 2066–2090 ) CrossRef.
- R. D. Ernst, A. Glöckner and A. M. Arif, Z. Kristallogr., 2007, 222, 333–334 CAS.
- J. Passmore and J. M. Rautiainen, Eur. J. Inorg. Chem., 2012, 6002–6010 CrossRef CAS.
- F. Weinhold and R. West, Organometallics, 2011, 30, 5815–5824 CrossRef CAS.
- F. Weinhold and R. West, J. Am. Chem. Soc., 2013, 135, 5762–5767 CrossRef CAS PubMed.
- H. Oberhammer and J. E. Boggs, J. Am. Chem. Soc., 1980, 102, 7241–7244 CrossRef.
- R. J. Gillespie and S. A. Johnson, Inorg. Chem., 1997, 36, 3031–3039 CrossRef CAS PubMed.
- S. Grabowsky, M. F. Hesse, C. Paulmann, P. Luger and J. Beckmann, Inorg. Chem., 2009, 48, 4384–4393 CrossRef CAS PubMed.
- T. S. Cameron, A. Decken, I. Krossing, J. Passmore, J. M. Rautiainen, X. Wang and X. Zeng, Inorg. Chem., 2013, 52, 3113–3126 CrossRef CAS PubMed.
- M. Ouchi, Y. Inoue, T. Kanzaki and T. Hakushi, Bull. Chem. Soc. Jpn., 1984, 57, 887–888 CrossRef CAS.
- Y. Inoue, M. Ouchi and T. Hakushi, Bull. Chem. Soc. Jpn., 1985, 58, 525–530 CrossRef CAS.
- D. J. Harrison, D. R. Edwards, R. McDonald and L. Rosenberg, Dalton Trans., 2008, 3401–3411 RSC.
- M. K. Skjel, A. Y. Houghton, A. E. Kirby, D. J. Harrison, R. McDonald and L. Rosenberg, Org. Lett., 2010, 12, 376–379 CrossRef CAS PubMed.
- K. Reuter, M. R. Buchner, G. Thiele and C. von Hänisch, Inorg. Chem., 2016, 55, 4441–4447 CrossRef CAS PubMed.
- M. Stürmann, W. Saak, M. Weidenbruch and K. W. Klinkhammer, Eur. J. Inorg. Chem., 1999, 579–582 CrossRef.
- C. Eaborn, P. B. Hitchcock, K. Izod and J. D. Smith, Angew. Chem., 1995, 107, 2936–2937 ( Angew. Chem., Int. Ed. Engl. , 1996 , 34 , 2679–2680 ) CrossRef.
- J. L. Atwood, S. G. Bott, C. M. Means, A. W. Coleman, H. Zhang and M. T. May, Inorg. Chem., 1990, 29, 467–470 CrossRef CAS.
- M. Barboiu and A. van der Lee, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2003, 59, m366–m368 CAS.
- M. Trömel, Z. Naturforsch., 2000, 55b, 243–247 Search PubMed.
- S. Chadwick and K. Ruhlandt-Senge, Chem. – Eur. J., 1998, 4, 1768–1780 CrossRef CAS.
- S. Chadwick, U. Englich and K. Ruhlandt-Senge, Inorg. Chem., 1999, 38, 6289–6293 CrossRef CAS PubMed.
- P. C. Junk and J. W. Steed, J. Chem. Soc., Dalton Trans., 1999, 407–414 RSC.
- Y. Y. Wei, B. Tinant, J. P. Declercq, M. Van Meerssche and J. Dale, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1988, 44, 73–77 CrossRef.
- T. B. Rubtsova, O. K. Kireeva, B. M. Bulychev, N. P. Streltsova, V. K. Belsky and B. P. Tarasov, Polyhedron, 1992, 11, 1929–1938 CrossRef CAS.
- P. Farina, W. Levason and G. Reid, Dalton Trans., 2013, 42, 89–99 RSC.
- J. Langer, S. Krieck, R. Fischer, H. Görls and M. Westerhausen, Z. Anorg. Allg. Chem., 2010, 636, 1190–1198 CrossRef CAS.
- D. Braga, M. Gandolfi, M. Lusi, D. Paolucci, M. Polito, K. Rubini and F. Grepioni, Chem. – Eur. J., 2007, 13, 5249–5255 CrossRef CAS PubMed.
- A. N. Chekhlov, Russ. J. Coord. Chem., 2009, 35, 492–495 CrossRef CAS.
- A. Verma, M. Guino-O, M. Gillett-Kunnath, W. Teng and K. Ruhlandt-Senge, Z. Anorg. Allg. Chem., 2009, 635, 903–913 CrossRef CAS.
- U. Englich, K. Ruhlandt-Senge and F. Uhlig, J. Organomet. Chem., 2000, 613, 139–147 CrossRef CAS.
- R. H. Crabtree, The Organometallic Chemistry of the Transition Metals, John Wiley & Sons, Ltd., Hoboken, NJ, 4th edn, 2005 Search PubMed.
- A. N. Swinburne and J. W. Steed, Podands. Supramolecular Chemistry: From Molecules to Nanomaterials, John Wiley & Sons, Ltd., see DOI:10.1002/9780470661345.smc058.
- P. C. Junk, L. M. Louis and M. K. Smith, Z. Anorg. Allg. Chem., 2002, 628, 1196–1209 CrossRef CAS.
- P. C. Junk and J. W. Steed, J. Coord. Chem., 2007, 60, 1017–1028 CrossRef CAS.
- W. Maudez and K. M. Fromm, Z. Anorg. Allg. Chem., 2012, 638, 1810–1819 CrossRef CAS.
- J. S. Alexander and K. Ruhlandt-Senge, Chem. – Eur. J., 2004, 10, 1274–1280 CrossRef CAS PubMed.
- M. R. Crimmin, A. G. M. Barrett, M. S. Hill, P. B. Hitchcock and P. A. Procopiou, Inorg. Chem., 2007, 46, 10410–10415 CrossRef CAS PubMed.
- K. Ruhlandt-Senge and U. Englich, Chem. – Eur. J., 2000, 6, 4063–4070 CrossRef CAS.
- B. Wei, Y. Tinant, M. Declercq, J. Van Meerssche and J. Dale, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1988, 44, 77–80 CrossRef.
- J. H. Burns, Inorg. Chim. Acta, 1985, 102, 15–21 CrossRef CAS.
- W. A. Wojtczak, M. J. Hampden-Smith and E. N. Duesler, Inorg. Chem., 1998, 37, 1781–1790 CrossRef CAS.
- T. S. Pochekutova, V. K. Khamylov, Y. A. Kurskii, G. K. Fukin and B. I. Petrov, Polyhedron, 2010, 29, 1381–1386 CrossRef CAS.
- M.-M. Zhao, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, m286–m286 CAS.
- L. Archer, M. J. Hampden-Smith and E. Duesler, Polyhedron, 1998, 17, 713–723 CrossRef CAS.
- H.-F. Ji, R. Dabestani, G. M. Brown and R. L. Hettich, J. Chem. Soc., Perkin Trans. 2, 2001, 585–591 RSC.
- S. Harder, B. Freitag, P. Stegner, J. Pahl and D. Naglav, Z. Anorg. Allg. Chem., 2015, 641, 2129–2134 CrossRef CAS.
- A. Steiner, G. T. Lawson, B. Walfort, D. Leusser and D. Stalke, J. Chem. Soc., Dalton Trans., 2001, 219–221 RSC.
- K. Reuter, G. Thiele, T. Hafner, F. Uhlig and C. von Hänisch, Chem. Commun., 2016, 52, 13265–13268 RSC.
- (a) Turbomole Version 7.0.1, Turbomole GmbH 2016. Turbomole is a development of University of Karlsruhe and Forschungszentrum Karlsruhe 1989–2007, Turbomole GmbH since 2007;; (b) F. Furche, R. Ahlrichs, C. Hättig, W. Klopper, M. Sierka and F. Weigend, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2014, 4, 91–100 CrossRef CAS.
- (a) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC; (b) F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057 RSC; (c) M. Dolg, H. Stoll, A. Savin and H. Preuss, Theor. Chim. Acta, 1989, 75, 173 CrossRef CAS; (d) H. Stoll, B. Metz and M. Dolg, J. Comput. Chem., 2002, 23, 767 CrossRef CAS PubMed; (e) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed; (f) S. Grimme, S. Ehrlich and I. Goerigk, J. Comput. Chem., 2011, 32, 1456 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2015, 71, 3–8 CrossRef PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- D. Kratzert, J. J. Holstein and I. Krossing, J. Appl. Crystallogr., 2015, 48, 933–938 CAS.
- Bruker, SADABS, Bruker AXS Inc., Madison, Wisconsin, USA, 2014 Search PubMed.
- Stoe & Cie, X-AREA and X-RED32, Stoe & Cie, Darmstadt, Germany, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1497467–1497471. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt04018g |
| This journal is © The Royal Society of Chemistry 2017 |