Open Access Article
Open Access ArticleIridium(III)-catalysed cross-linking of polysiloxanes leading to the thermally resistant luminescent silicone rubbers†
Regina M.
Islamova
 *a,
Mikhail V.
Dobrynin
a,
Andrey V.
Vlasov
a,
Anzhelika A.
Eremina
a,
Mikhail A.
Kinzhalov
*a,
Mikhail V.
Dobrynin
a,
Andrey V.
Vlasov
a,
Anzhelika A.
Eremina
a,
Mikhail A.
Kinzhalov
 a,
Ilya E.
Kolesnikov
a,
Andrey A.
Zolotarev
a,
Eleonora A.
Masloborodova
c and
Konstantin V.
Luzyanin
a,
Ilya E.
Kolesnikov
a,
Andrey A.
Zolotarev
a,
Eleonora A.
Masloborodova
c and
Konstantin V.
Luzyanin
 *ab
*ab
aSaint Petersburg State University, 7/9, Universitetskaya nab, Saint Petersburg, 199034 Russia. E-mail: r.islamova@spbu.ru
bDepartment of Chemistry, University of Liverpool, Crown Street, Liverpool L69 7ZD, UK. E-mail: konstantin.luzyanin@liverpool.ac.uk
cLebedev Research Institute for Synthetic Rubber, Gapsal'skaya, 1, 198035 Saint Petersburg, Russian Federation
First published on 1st November 2017
Abstract
Cyclometallated iridium(III) complexes with 2-phenylpyridine [Ir(ppy)2(CNR)Cl] (ppy = (2-phenylpyridinato-C2,N), R = Xyl, Mes), [Ir(ppy)2(CNR)2](OTf) (R = Xyl, Mes), and fac-[Ir(ppy)3] catalyse the cross-linking of polysiloxanes exclusively at temperatures above 100 °C leading to luminescent rubbers.
In the past decades, silicone-based technology has evolved from specialty, high-performance applications into broad industrial usage.1 Cured silicone coatings have substantial thermal, weather corrosion, biofouling, and abrasion resistance.2 These coatings accounted for almost three quarters of contemporary paper and film coating production;3 they are also used for metal3b and glass protection,4 and as substrates for pressure-sensitive adhesives.5
The conventional preparation of silicone coating involves metal-catalysed hydrosilylative cross-linking (curing) of vinyl- and hydrogen-functional poly(dimethylsiloxanes).2c,3a,6 Ideally, coating composition should not gel at RT but cure rapidly at a temperature above 100 °C allowing the coated substrate to be further processed without cooling down.3a Although these requirements can be addressed by application of a suitable catalyst, the commonly used Karstedt's catalyst (a platinum(0) complex with 1,3-divinyl-1,1,3,3-tetramethyldisiloxane, Fig. 1a) shows limited chemical and thermal stability and excessive activity. The use of inhibitors is required to prevent immediate cross-linking even at RT; therefore, its application at higher temperatures is not straightforward.7 Marciniec et al.7 first reported iridium(I)-based siloxide catalysts (Fig. 1b) that cure siloxanes at ca. 200 °C; however, the properties of the thus prepared rubbers were not rationalized.
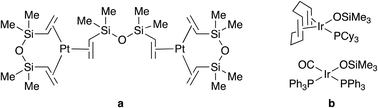 | ||
| Fig. 1 (a) Karstedt's platinum-disiloxane and (b) Marciniec's iridium–siloxide catalysts for hydrosilylative silicone curing. | ||
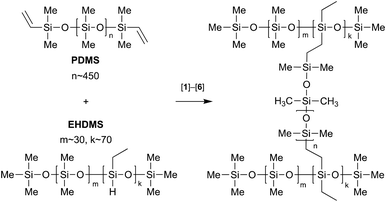
In the current study, we scrutinised a series of alternative iridium(III) complexes 1–6 (Scheme 1) as catalysts for the cross-linking of α,ω-di(vinyldimethylsiloxy)poly(dimethylsiloxane) (PDMS) with trimethylsilyl-terminated poly(dimethylsiloxane-co-ethylhydrosiloxane) (EHDMS). We established the temperature-curing profile for these complexes and evaluated the properties of the silicone compositions obtained. In addition, we measured the luminescence emission profiles of 2–6 and of the corresponding rubbers prepared, and developed a procedure for immediate measurement of the coating thickness based upon optical detection.
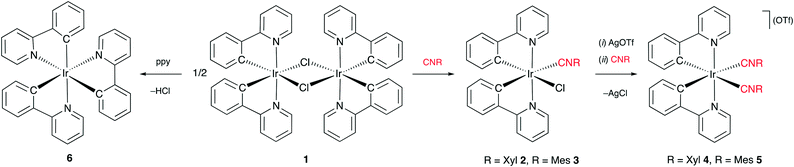
Synthesis and characterization of catalysts
Iridium(III) complexes, [Ir(ppy)2(CNR)Cl] (ppy = (2-phenylpyridinato-C2,N), R = Xyl 2, Mes 3) and [Ir(ppy)2(CNR)2](OTf) (R = Xyl 4, Mes 5; OTf = CF3SO3−), were prepared from a chloro-bridged dimer, [Ir(ppy)2(μ-Cl)]2 (1, Scheme 1). Thus, the reaction of 1 with two equiv. of isocyanide CNR (R = Xyl, R = Mes) in CH2Cl2 at RT afforded 2 or 3 in 82–85% isolated yield. Further reaction of 1 equiv. of 2 or 3 with 1 equiv. of CNR in the presence of 1 equiv. of AgOTf in CH2Cl2 at RT gave 4 or 5 in 74–85% yield. Complex fac-[Ir(ppy)3] (6) was prepared via a known procedure starting from 1 and ppy.8 Complexes 2–5 were obtained as pale yellow (2 and 3) or colourless (4 and 5) air- and moisture-stable solids. The detailed characterization of new species 2, 3, and 5 using elemental analyses (C, H, N), HR-ESI+-MS, FT-IR, 1D (1H, 13C{1H}) and 2D (1H,1H-COSY, 1H,13C-HMQC/1H,13C-HSQC, 1H,13C-HMBC) NMR spectroscopy is provided in the ESI.† The spectral data for the known species 1, 4 and 6 (1H and 13C{1H} NMR) were in agreement with the data reported.8,9The structures of 2, 3 and 5 were additionally elucidated by single-crystal X-ray diffraction (see Tables S1 and S2 and Fig. S1 and S2†). In all complexes, the iridium(III) centre has a distorted octahedral coordination environment, and the two nitrogen atoms of the phenylpyridine ligands are placed in trans-position to each other. The bond distances and angles in 2, 3, and 5 are comparable to those in the known iridium isocyanide complexes (see the ESI† for details).9a,10
Iridium(III)-catalysed cross-linking
Iridium-catalysed hydrosilylative cross-linking was studied on model PDMS/EHDMS mixtures with catalysts 1−6 pre-dissolved in them (10−3−10−5 M, Scheme 2). The course of cross-linking was monitored by DSC through the measurement of the heat effect of the process. At the temperatures below 80 °C, 1−6 did not demonstrate any visible catalytic activity. In addition, we found that the solutions of 1−6 in the siloxane mixture could be stored at RT for at least one year without decomposition.The curing properties of 1–6 were evaluated in the 80–180 °C temperature range allowing the identification of the curing time (τcuring) and the enthalpy (ΔH) of the cross-linking process. Representative data (catalyst concentration 1.0 × 10−4 M) are included in Table 1, while full data are provided in Table S3 in the ESI.† Catalysts 1−6 started to work at ca. 125 °C, while the optimal temperature range was found to be 150−180 °C. Complexes 2, 3 and 6 were the most efficient, giving the shortest τcuring of 5–50 min at 1.0 × 10−4 M catalyst concentration; catalysts 1, 4 and 5 were substantially less active at 150−180 °C. Longer curing times were observed at a lower catalyst concentration (1.0 × 10−5 M). Although we found that an increase of the concentration to 1.0 × 10−3 M shortens further the curing times, it greatly increases the catalyst consumption (Table S3†).
| Catalysta | τ curing, min at a selected temperatureb (°C) | ΔH, J g−1 | ||||
|---|---|---|---|---|---|---|
| 80 | 100 | 125 | 150 | 180 | ||
| a Catalyst concentration used 1.0 × 10−4 M. b PDMS 100 poise. c No curing observed after 24 h. | ||||||
| 1 | —c | —c | 300 | 100 | 45 | −1.3 |
| 2 | —c | 360 | 70 | 50 | 15 | −0.8 |
| 3 | —c | 300 | 70 | 45 | 15 | −0.8 |
| 4 | —c | —c | 300 | 107 | 20 | −1.8 |
| 5 | —c | —c | 630 | 147 | 30 | −1.9 |
| 6 | —c | —c | 390 | 45 | 5 | −1.7 |
According to DSC, the average peak temperature (Fig. S3†) for the representative catalysts 2 and 3 (1.0 × 10−4 M) was 155 °C, the average onset peak temperature was 140 °C and the endset peak temperatures were 205 (for 2) and 190 °C (for 3), respectively. The enthalpy values of the cross-linking were −0.8 J g−1 for both 2 and 3.
Properties of silicone compositions formed
Silicone formulations obtained with catalysts 1−6 incorporate no structural defects, i.e. no bubbles and the surface of the silicone rubbers is uniform. All iridium catalysts 1–6 increased the thermal stability of the obtained rubbers (Table S4†). In air, the temperature of the initial weight loss of the silicone rubber obtained with 1–6 is higher by 80–120 °C, when compared to that obtained with the Karstedt's catalyst used as a benchmark. Under argon, temperature of the initial weight loss of the silicone rubber obtained with 1 and 2 is higher by 35–45 °C when compared to a reference. Rubbers obtained with 3–6 showed comparable or lower temperature of the initial weight loss.The final residues of all rubbers (upon heating for up to 800 °C) were ca. 3–7% and 51–54% in argon and in air, respectively. Different results obtained in air and argon are expected and can be partially rationalized by the mechanism of thermal degradation proposed earlier.11 In our previous study,6e,f [PtCl2(NCCH2Ph)2] used as the catalyst increased the temperature of the initial weight loss by 30–40 or 80–90 °C against the Karstedt's catalyst in air or argon, respectively. Hence, iridium complexes 1–6 produce silicone formulations that are far more thermally stable in air than the Karstedt's catalyst or [PtCl2(NCCH2Ph)2]. The thermal stability of the silicone rubber depended also on the temperature during the cross-linking and the concentration of the catalyst (Fig. S4 and S5 and Tables S4–S6†). The rubbers prepared at higher temperatures and with greater catalyst loading showed a higher temperature of the initial weight loss and a larger final residue due to a plausible increase of the degree of cross-linking in this case.11,12
Swelling measurements provided information about soluble fraction (wsol) and polymer volume fraction (υ). Silicone compositions obtained with 100 poise PDMS using representative catalysts 2 and 3 (1.0 × 10−5 M) had a wsol of 13.3% (for 2) and 10.7 (for 3), and a υ of 0.2 (for both catalysts). The soluble fractions were larger than those in the case of rubbers obtained with both the Karstedt's catalyst (1.0 × 10−5 M, wsol 5.1%, υ 0.22), and [PtCl2(PhCH2CN)2] (1.0 × 10−5 M, wsol 5.8%, υ 0.21).6e,f Interestingly, the silicone compositions obtained with the less active catalyst 5 possess wsol 2.7% and υ 0.2, indicative of a higher degree of cross-linking . Tensile tests for elastic properties suggested that the silicone rubbers prepared have similar elongation at break (L) and tensile strength (σ) to the rubbers obtained with the Karstedt's catalyst.6e,f
Photophysical studies
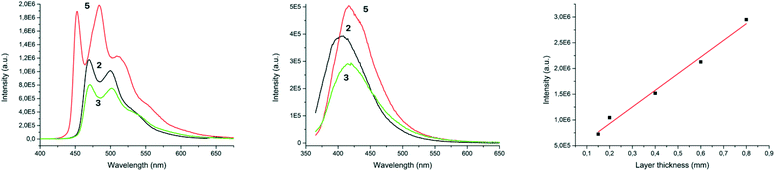
As the next step, we evaluated the luminescence properties of new complexes 2, 3, and 5 (as 1.0 × 10−4 M solutions in CH2Cl2) and those of the silicone compositions prepared using these catalysts (Fig. 2, S8–S11, Tables S5 and S6†). The photophysical properties of 4 and 6 have been previously reported.8,9Among the new species 2, 3, and 5, the strongest phosphorescence was observed for complexes 2 and 3 followed by 5 with two maxima for each (470 and 502 nm for 2, 472 and 502 nm for 3, and 453 and 484 nm for 5, Fig. 2a). This agrees well with the data reported for complexes 4 and 6, where the maxima were observed at 454 and 486 nm (for 4) and at 510 nm (for 6).8,9 Maximum phosphorescence quantum yields (Φ) CH2Cl2 were achieved with 2 (43%), and 3 (41%); they are twice as large when compared to those of 5 (23%) and the known complex 4 (24%), and are akin to 6 (40%). The lifetimes (τ) were in the range of 1.5–1.9 μs. Complex 1 does not exhibit luminescence under the conditions of this study.
The prepared silicone compositions showed different luminescence profiles (Fig. 2b). Herein, a strong shortwave shift is evident, and for all the samples, only one broad emission wavelength is observed (e.g. at 417 nm for rubbers prepared with 3). The overall emission intensity for the cross-linked silicone rubbers has also substantially decreased when compared to that of 2–6 in solution. The quantum yield cannot be carefully estimated for cured rubbers because of the complexity of calculations and polymer self-absorption.
One of the major problems in the polymer industry concerns the immediate measurement of coating thickness, which is typically achieved using mechanical tools, while the application of methods based on the reading of the optical density of luminescence intensity is limited.13 In the course of this study, we prepared a series of thin films (0.1–1.0 mm) using representative catalyst 3, and measured their luminescence at 408 nm in reflectance mode. The plot of the luminescence intensity as a function film thickness is given in Fig. 2c. A linear fit was applied for all the points between 0.15–0.80 mm, where good correlation coefficient (Pearson correlation coefficient 0.99457) was achieved. For samples thinner than 0.15 mm and thicker than 1.0 mm, the intensity reading values do not follow a linear dependence. Our measurements clearly indicate that the thickness of the polymer coating prepared with catalysts 2–6 can easily be monitored using optical detection on the basis of the Beer–Lambert–Bouguer law, and that this approach might be further explored towards industrial applications.
To conclude, we discovered that cyclometallated iridium(III) complexes with 2-phenylpyridine 1–6 show a unique temperature-curing profile in the cross-linking of PDMS and EHDMS. Maximum efficiency is achieved in the 150–180 °C temperature range, where curing is completed within 5–50 min. Complexes 1–6 demonstrated dissimilar activity with [Ir(ppy)2(CNR)Cl] (2 and 3) and fac-[Ir(ppy)3] (6) being more active than the others. The silicone compositions prepared with 1–6 showed thermal stability in air superior to those achieved with the benchmark Karstedt's catalyst and comparable mechanical properties. Complexes 2–6 and silicone rubbers prepared using those exhibit luminescence properties enabling the measurement of the thickness of the coating using optical detection. Further studies on the design of new metal catalysts for siloxane cross-linking and the identification of their mechanism of action are currently underway in our group.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Preparation of polymer compositions using new metal catalysts and assessment of their properties were supported by the Russian Ministry of Education and Science (State Contract 14.W03.31.0014, Megagrant for RMI). Design, preparation and characterization of iridium organometallics were funded by the Russian Foundation for Basic Research (grant 16-33-60123 mol_a_dk for MAK). AVV is much obliged to Saint Petersburg State University for a postdoctoral fellowship (12.50.1188.2014), while KVL additionally thanks the University of Liverpool for supporting his research activities. Measurements were performed at the Center for Magnetic Resonance, Center for X-ray Diffraction Studies, Center for Chemical Analysis and Materials Research, Center of Thermal Analysis and Calorimetry, Center for Optical and Laser Materials Research, “Geomodel” Research Center and Chemistry Educational Centre (all in Saint Petersburg State University).Notes and references
- (a) G. L. Witucki, J. Coat. Technol., 1993, 65, 57–60 CAS; (b) Paint and Coating Testing Manual, ed. J. V. Koleske, ASTM International, West Conshohocken, PA, USA, 2012 Search PubMed.
- (a) H.-H. Moretto, M. Schulze and G. Wagner, Silicones in Ullmann's Encyclopedia of Industrial Chemistry, Wiley–VCH, Weinheim, 1993, vol. A 24, pp. 57–93 Search PubMed; (b) D. Chen, F. Chen, X. Hu, H. Zhang, X. Yin and Y. Zhou, Compos. Sci. Technol., 2015, 117, 307–314 CrossRef CAS; (c) D. Wang, J. Klein and E. Mejía, Chem. – Asian J., 2017, 12, 1180–1197 CrossRef CAS PubMed; (d) A. Kottmann, E. Mejía, T. Hémery, J. Klein and U. Kragl, Chem. – Asian J., 2017, 12, 1168–1179 CrossRef CAS PubMed.
- (a) D. Troegel and J. Stohrer, Coord. Chem. Rev., 2011, 255, 1440–1459 CrossRef CAS; (b) Y. Liu, G.-P. Yan, H.-W. Che, X.-Y. Wang and Q.-Z. Guo, J. Appl. Polym. Sci., 2011, 119, 1156–1160 CrossRef CAS; (c) K. W. Chew, A. H. Yahaya and A. K. Arof, Pigm. Resin Technol., 2000, 29, 364–368 CrossRef CAS; (d) M. Lejars, A. Margaillan and C. Bressy, Chem. Rev., 2012, 112, 4347–4390 CrossRef CAS PubMed; (e) D. C. Webster and B. J. Chisholm, New directions in antifouling technology in Biofouling, Wiley-Blackwell, Oxford, 2012, pp. 366–387 Search PubMed; (f) D. E. Katsoulis, M. Suto and N. Kushibiki, Free-standing films based on silicone resins, San Jose, CA, USA, 2003 Search PubMed.
- (a) B. I. Gutek and B. VanWert, Ind. Eng. Chem. Prod. Res. Dev., 1982, 21, 601–604 CrossRef CAS; (b) N. Anderson and B. Zhu, US8277945 B2, 2012.
- M. Andriot, S. H. Chao, A. Colas, S. Cray, F. D. Buyl, J. V. DeGroot, A. Dupont, T. Easton, J. L. Garaud, E. Gerlach, F. Gubbels, M. Jungk, S. Leadley, J. P. Lecomte, B. Lenoble, R. Meeks, A. Mountney, G. Shearer, S. Stassen, C. Stevens, X. Thomas and A. T. Wolf, Silicones in Industrial Applications in Inorganic Polymers, ed. R. de Jaeger and M. Gleria, Nova Science Publishers, Hauppauge NY, USA, 2007, ch. 2, pp. 61–161 Search PubMed.
- (a) Hydrosilylation, A Comprehensive Review on Recent Advances, ed. B. Marciniec, Springer Dordrecht, Netherlands, 2009 Search PubMed; (b) A. K. Roy, A Review of Recent Progress in Catalyzed Homogeneous Hydrosilation in Advances in Organometallic Chemistry, ed. W. Robert, A. Hill and J. M. Fink, Academic Press, Cambridge, MA, USA, 2007, vol. 55, pp. 1–59 Search PubMed; (c) B. Marciniec, A. Kownacka, I. Kownacki, M. Hoffmann and R. Taylor, J. Organomet. Chem., 2015, 791, 58–65 CrossRef CAS; (d) M. Y. Demakova, D. S. Bolotin, N. A. Bokach, R. M. Islamova, G. L. Starova and V. Y. Kukushkin, ChemPlusChem, 2015, 80, 1607–1614 CrossRef CAS; (e) R. M. Islamova, M. V. Dobrynin, D. M. Ivanov, A. V. Vlasov, E. V. Kaganova, G. V. Grigoryan and V. Y. Kukushkin, Molecules, 2016, 21, 311–321 CrossRef PubMed; (f) R. M. Islamova, A. V. Vlasov, M. V. Dobrynin, E. A. Masloborodova and E. V. Kaganova, Russ. J. Gen. Chem., 2015, 85, 2609–2613 CrossRef CAS.
- I. Kownacki, B. Marciniec, K. Szubert, M. Kubicki, M. Jankowska, H. Steinberger and S. Rubinsztajn, Appl. Catal., A, 2010, 380, 105–112 CrossRef CAS.
- A. B. Tamayo, B. D. Alleyne, P. I. Djurovich, S. Lamansky, I. Tsyba, N. N. Ho, R. Bau and M. E. Thompson, J. Am. Chem. Soc., 2003, 125, 7377–7387 CrossRef CAS PubMed.
- (a) A. Maity, L. Q. Le, Z. Zhu, J. Bao and T. S. Teets, Inorg. Chem., 2016, 55, 2299–2308 CrossRef CAS PubMed; (b) Y. K. Radwan, A. Maity and T. S. Teets, Inorg. Chem., 2015, 54, 7122–7131 CrossRef CAS PubMed.
- (a) N. M. Shavaleev, F. Monti, R. Scopelliti, N. Armaroli, M. Grätzel and M. K. Nazeeruddin, Organometallics, 2012, 31, 6288–6296 CrossRef CAS; (b) M. A. Kinzhalov, A. A. Eremina, D. M. Ivanov, A. S. Novikov, E. A. Katlenok, K. P. Balashev and V. V. Suslonov, Z. Kristallogr. - Cryst. Mater., 2017 DOI:10.1515/zkri-2017-2065 , in press.
- S. Hamdani, C. Longuet, D. Perrin, J.-M. Lopez-cuesta and F. Ganachaud, Polym. Degrad. Stab., 2009, 94, 465–495 CrossRef CAS.
- E. Delebecq, S. Hamdani-Devarennes, J. Raeke, J.-M. L. Cuesta and F. Ganachaud, ACS Appl. Mater. Interfaces, 2011, 3, 869–880 CAS.
- (a) A. Revis and M. J. Ziemelis, US5107008, 1992; (b) K. Murugesan, K. Sivasubramanian, I. Ramakrishnan and V. Khare, WO2016/109542, 2016; (c) M. A. Roe and T. P. Hunter, WO2013/084006, 2013; (d) D. E. Wolfe, US2011/0171062, 2011.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures for catalysts preparation and characterization, description of curing experiments, studies on the properties of prepared polymers and photophysical data. CCDC 1508596, 1505628 and 1505626. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cy02013a |
| This journal is © The Royal Society of Chemistry 2017 |