A family of solar light responsive photocatalysts obtained using Zn2+ Me3+ (Me = Al/Ga) LDHs doped with Ga2O3 and In2O3 and their derived mixed oxides: a case study of phenol/4-nitrophenol decomposition†
Abstract
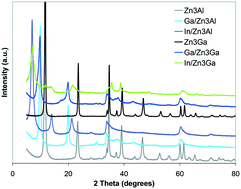
Photocatalytic decomposition of dangerous organic contaminants using irradiation with sunlight is of great importance for environmental remediation and human health. In this paper Zn2+Me3+ (Me = Al/Ga) layered double hydroxides (LDHs) doped with gallium oxide (Ga2O3) and indium oxide (In2O3) and their derived mixtures of mixed oxides (MMOs) as novel solar light driven photocatalysts for degrading a mixture of phenol/4-nitrophenol (Ph–4NPh) are presented. The Ga2O3/ZnMeLDHs and In2O3/ZnMeLDHs were fabricated by exploiting the LDH capability to rebuild its structure, after being destroyed by calcination at 550 °C, in aqueous solutions of gallium sulfate [Ga2(SO4)3] and indium acetate [In(C2H3O2)3], respectively, while the corresponding MMOs were obtained after the calcination at 750 °C. The effects on the structure, surface characteristics and photo-absorption properties of the catalysts were assessed using X-ray diffraction, energy dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy, Fourier-transform infrared spectroscopy and ultraviolet-visible analyses. The findings demonstrate that both Ph and 4NPh are degraded by both the reconstructed LDHs and their derived MMOs in such a way that an increased efficiency is obtained for In/Zn3Ga_750 and Ga/Zn3Ga_750 which degraded almost 90% of the 4NPh. In comparison, for In/Zn3Ga and Ga/Zn3Ga catalysts, the degradation efficiency reached only 69% and 47%, respectively. Furthermore, in the family of ZnAlLDH derived catalysts, In/Zn3Al_750 is able to decompose 98% of 4-NPh and 77% of Ph, after 240 min of irradiation, whereas the efficiency of Ga/Zn3Al_750 reached only 57% for 4-NPh and 39% for Ph. The as-prepared LDHs, Zn3Al and Zn3Ga, are almost inactive for degradation of a Ph–4NPh mixture under solar light.
- This article is part of the themed collection: Catalytic reactivity of surfaces: in recognition of François Gault

 Please wait while we load your content...
Please wait while we load your content...