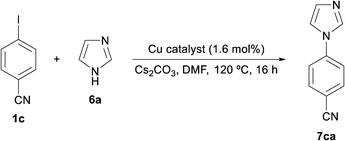
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of C–C, C–S and C–N bonds catalysed by supported copper nanoparticles†
Alexander Yu.
Mitrofanov
 a,
Arina V.
Murashkina
a,
Iris
Martín-García
a,
Arina V.
Murashkina
a,
Iris
Martín-García
 b,
Francisco
Alonso
b,
Francisco
Alonso
 *b and
Irina P.
Beletskaya
*b and
Irina P.
Beletskaya
 *a
*a
aDepartment of Chemistry, Moscow State University, Leninskie Gory, GSP-1, Moscow 119991, Russia. E-mail: beletska@org.chem.msu.ru
bInstituto de Síntesis Orgánica (ISO) and Departamento de Química Orgánica, Facultad de Ciencias, Universidad de Alicante, Apdo. 99, 03080 Alicante, Spain. E-mail: falonso@ua.es
First published on 16th August 2017
Abstract
Transition-metal catalysed cross-coupling reactions are still dominated by palladium chemistry. Within the recent past, copper has gained ground against palladium by virtue of its cheaper price and equivalent function in certain reactions. Four catalysts consisting of copper nanoparticles on zeolite, titania, montmorillonite and activated carbon have been tested in three palladium- and ligand-free cross-coupling reactions to form carbon–carbon, carbon–sulfur and carbon–nitrogen bonds. CuNPs/zeolite has been found to be the best one in the Sonogashira reaction of aryl iodides and arylacetylenes, as well as in the coupling of aryl halides with aryl and alkyl thiols, being reusable in both cases. However, the arylation of nitrogen-containing heterocycles (imidazole, pyrazole, benzimidazole and indole) has been better accomplished with CuNPs/titania, albeit CuNPs/activated carbon showed better recycling properties. The catalytic activity of the nanostructured catalysts has been compared with that of twelve commercial copper catalysts, with the former outperforming the latter in the three types of reactions studied.
Introduction
The copper-catalysed formation of carbon–carbon and carbon–heteroatom bonds is not only a simple renaissance of Ullmann's chemistry; it is a new area of transition-metal catalysed reactions where copper sometimes competes with palladium and often reveals quite different behaviour.1 There are many Cu(I) and Cu(II) derivatives, such as salts, oxides, or complexes with ligands which are widely used in catalysis. Even though many of these catalytic systems helped to overcome some of the main drawbacks of traditional copper-promoted procedures, the homogeneous nature of this catalysis hampers the recovery and reuse of these catalysts and their practical applicability,2 mainly in the synthesis of drug molecules,3 which must be free of any residual metal. Consequently, heterogeneous copper catalysis has attracted a great deal of attention in recent years. There are many examples in the literature about the utility of heterogeneous copper-based catalysts in cross-coupling reactions, most of them based on copper complexes with functionalised ligands immobilised on different supports.4 Still, these catalytic systems require the synthesis of specialised ligands, immobilisation and copper complex formation steps that, often, may catalyse a narrow scope of reactions. In this sense, catalytic systems which rely on Cu nanoparticles (CuNPs) may be good alternatives to immobilised complexes because of their high surface-to-volume ratio, which provides them with higher reactivity and selectivity when compared with bulk catalysts.5 Although it is difficult to separate nanoparticles using standard methods due to their nanometric size,6a immobilisation on inorganic supports not only favours their stabilisation and dispersion but separation from the reaction medium.6b–fImmobilised copper nanoparticles obtained by various methods and on different supports have been demonstrated to be versatile and reusable catalysts for a wide range of reactions, including cross-coupling reactions forming carbon–carbon and carbon–heteroatom bonds (C–N, C–O, C–S, C–P),4,7 oxidative coupling reactions,8 as well as multicomponent reactions.9
In this work, we set out a broad synthetic application of some copper-based nanocatalysts supported on four different materials: a carbonaceous material (activated carbon),10 a ceramic metal oxide (nanosized titania),11 a clay mineral (montmorillonite-K10)12 and a microporous zeolite (sodium Y zeolite).13 In particular, we have already demonstrated the great versatility of activated carbon14 and titania15 as supports for metal nanoparticles in catalytic organic reactions. Herein, the support-dependent catalytic behaviour has been evaluated in the Sonogashira–Hagihara reaction and in the coupling of aryl halides with thiols and azoles. To the best of our knowledge, such a comparative study involving four supports of different nature has never been reported for these type of reactions.
Results and discussion
Catalyst preparation and characterisation
The CuNP-based catalysts were prepared following the arene-catalysed16 chemical reduction of metal salts, as follows;9b anhydrous CuCl2 was rapidly reduced with lithium metal and a catalytic amount of 4,4′-di-tert-butylbiphenyl (DTBB) in THF at room temperature, followed by the addition of the support. The resulting mixture was filtered, washed and dried.9bThe full characterisation of copper nanoparticles on activated carbon (CuNPs/C)9b and copper nanoparticles on zeolite Y (CuNPs/ZY)8b was already reported in the literature (see also the ESI†). The copper nanoparticles on titania (CuNPs/TiO2) and copper nanoparticles on montmorillonite (CuNPs/MK-10)12b were characterised by means of transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS) (see the ESI†). In general, all the catalysts unveiled the presence of well dispersed spherical nanoparticles on the supports, with average sizes in the range of 1–6 nm. Analysis by XPS revealed that the surface of the CuNPs in all the catalysts is oxidised and consists of both Cu(I) and Cu(II) oxides for CuNPs/C and CuNPs/ZY, mainly Cu(I) oxide for Cu/TiO2 and Cu(II) oxide for CuNPs/MK-10. The following copper loadings and BET areas were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES) and adsorption isotherms, respectively, for the different catalysts: CuNPs/C (3.5 wt%, 1224 m2 g−1), CuNPs/TiO2 (1.9 wt%, 119 m2 g−1), CuNPs/ZY (3.0 wt%, 621 m2 g−1) and CuNPs/MK-10 (1.7 wt%, 89 m2 g−1).
The Sonogashira–Hagihara reaction
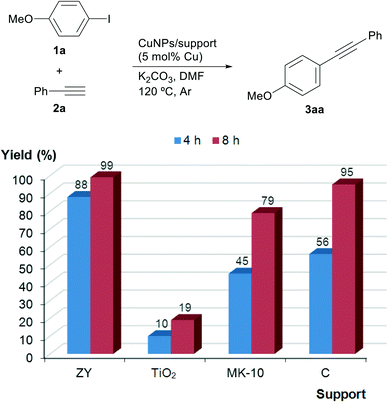
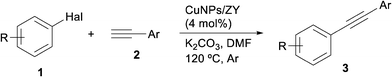
The Sonogashira–Hagihara reaction can be considered to be one of the most widely practiced strategies to synthesise alkyl and aryl acetylenes as well as conjugated enynes. In this scenario, palladium has always occupied a leading position as the catalytic metal.17 Latterly, copper has emerged as a competitive cheaper alternative to palladium, providing in many cases comparable results with simple catalytic systems.18In order to investigate the effect of the nature of the support in the Cu-catalysed Sonogashira coupling, 4-iodoanisole (1a) and phenylacetylene (2a) were chosen as the model substrates. A preliminary screening which considered the catalyst loading, base, solvent and temperature as the variables allowed us to conclude that 5 mol% copper loading and K2CO3 as the base in DMF at 120 °C were appropriate conditions for comparative purposes of all the catalysts. As expected, the catalytic activity of the nanoparticles was found to depend on the nature of the support with significant differences and the following decreasing order of activity: CuNPs/ZY > CuNPs/C > CuNPs/MK-10 > CuNPs/TiO2 (Fig. 1). The higher activity of CuNPs/ZY and CuNPs/C might be, tentatively, correlated with the larger surface area of these supports and the presence of both Cu(I) and Cu(II) in the catalysts.
The most active catalyst (CuNPs/ZY) was deployed in the coupling reaction of phenylacetylene with different aryl halides at a ca. 4 mol% loading [determined from the Cu content (3.0 wt%) and the Cu2O/CuO area from XPS (ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)] (Table 1).
1)] (Table 1).
| Aryl halide | t (h) | Product | Yieldb (%) |
|---|---|---|---|
| a Aryl halide (1, 0.25 mmol), arylacetylene (2, 1.5 equiv.), CuNPs/ZY (ca. 4 mol%) and K2CO3 (0.5 mmol), DMF (1 mL), 120 °C, Ar. b 1H NMR yield. c Reaction at 150 °C. d Isolated yield. | |||
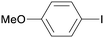 1a
1a
|
8 |
 3aa
3aa
|
99 |
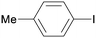 1b
1b
|
8 |
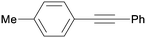 3ba
3ba
|
99 |
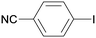 1c
1c
|
4 |
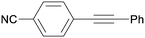 3ca
3ca
|
98 |
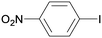 1d
1d
|
2 |
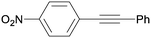 3da
3da
|
95 |
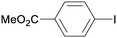 1e
1e
|
2 |
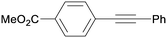 3ea
3ea
|
98 |
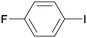 1f
1f
|
4 |
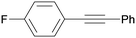 3fa
3fa
|
96 |
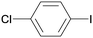 1g
1g
|
4 |
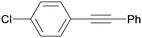 3ga
3ga
|
98 |
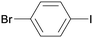 1h
1h
|
4 |
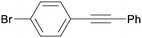 3ha
3ha
|
98 |
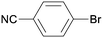 1i
1i
|
8 |
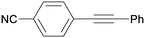 3ca
3ca
|
25 |
| 8c | 60cd | ||
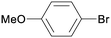 1j
1j
|
8 |
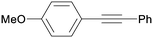 3aa
3aa
|
0 |
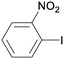 1k
1k
|
2 |
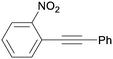 3ka
3ka
|
99 |
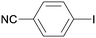 1c
1c
|
24c |
 3cb
3cb
|
40d |
 1c
1c
|
24c |
 3cc
3cc
|
61d |
 1c
1c
|
24c |
 3cd
3cd
|
90d |
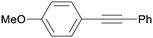
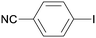
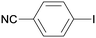
All reaction products were formed in a selective manner with almost quantitative yields from aryl iodides substituted either with electron-withdrawing or electron-donating groups (3aa–3ea). As expected, electron-poor aryl iodides were shown to be more reactive than those bearing electron-donating groups in the aromatic ring (compare the reaction times of 1a and 1b with those of 1c–1e and 1k). The reaction with haloaryl iodides was highly chemoselective towards the C–I bond (3fa–3ha). Unfortunately, this catalyst was confirmed to be less efficient in the coupling of aryl bromides (1i and 1j) under the standard conditions. The electronic effect on the more reluctant to react substituted arylacetylenes was also analysed via reaction with 4-iodobenzonitrile (1c): the lower the electron-rich character of the arylacetylene, the better the yield obtained (3cb–3cd).
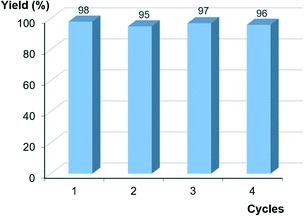
The heterogeneous nature of CuNPs/ZY facilitated its recovery by centrifugation and recycling. Indeed, the catalyst showed an excellent performance when reused in four consecutive cycles, in the standard reaction of 4-iodoanisole (1a) and phenylacetylene (2a) (Fig. 2), with no discernible variation in particle size after the fourth cycle (Fig. S5, ESI†). The hot filtration test after the first run disclosed a leaching of 0.14% of the original copper content (0.01% Cu after the fourth run), as determined by ICP-MS; this leached copper was found to be catalytically inactive.
By comparing the catalytic activity of CuNPs/ZY with that of other catalysts reported in the literature, we can conclude that its activity and that reported by Rothenberg et al. using copper clusters stabilised by tetra-butylammonium acetate (5 mol% Cu, DMF, 110 °C, 24 h) are alike,19ae.g., similar reactivity towards various aryl halides. Commercial nano-CuO19b manifested lower activity than CuNPs/ZY, given the higher catalyst loading and temperature required for the coupling of aryl iodides (10 mol%, DMSO, 160 °C, 12 h). Neither of the two aforementioned catalysts were reutilised. CuNPs/ZY was not active in the reaction of aryl chlorides with acetylenes, in contrast with Cu(0)NPs/Al2O3 which could catalyse this reaction at room temperature.7a The absence of an oxide film on the copper surface seems to be crucial for this enhanced reactivity. Indeed, CuO/Al2O3 not only exhibited relatively lower activity but also was evinced to be non-recyclable, with a copper loss of 63% after the first cycle.19c Most importantly, CuNPs/ZY is a clear alternative to PdNPs stabilised by a tris-imidazolium salt20a and PdNPs/DNA,20b which were previously described by us, though the latter was far more active in the reaction with aliphatic alkynes under milder conditions.
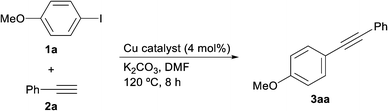
We believe that every laboratory-made catalyst should be more efficient than commercial catalysts used for the same purpose in order to justify the time, materials and human resources employed during its preparation. With this principle in mind, we compared the catalytic activity of CuNPs/ZY with that of a wide variety of commercial copper sources in the coupling reaction of 4-iodoanisole (1a) and phenylacetylene (2a) (Table 2). We were delighted to demonstrate that our catalyst was distinctly superior to the commercial catalysts tested. Only CuOTf and Cu(OTf)2, the most expensive substances in Table 2, furnished the coupling product in moderate conversion albeit with the concomitant formation of substantial amounts of the alkyne homocoupling product (1,4-diphenylbuta-1,3-diyne).21 It is worthwhile mentioning that in the case of our Sonogashira reactions the diyne by-product, if present, was formed in negligible amounts.
| Entry | Catalyst | Conversionb (%) |
|---|---|---|
a
1a (0.25 mmol), 2a (1.5 equiv.), Cu catalyst (4.0 mol%) and K2CO3 (0.5 mmol), DMF (1 mL), 120 °C, Ar, 8 h.
b Conversion into 3aa determined by GLC based on 1a.
c The alkyne homocoupling side product 1,4-diphenylbuta-1,3-diyne and 3aa were obtained in a ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. 2 ratio.
|
||
| 1 | Cu(0) | 0 |
| 2 | Cu2O | 8 |
| 3 | CuO | 0 |
| 4 | CuCl | 5 |
| 5 | CuCl2 | 8 |
| 6 | CuBr | 0 |
| 7 | CuI | 4 |
| 8 | CuOAc | 9 |
| 9 | Cu(OAc)2 | 7 |
| 10 | CuOTf | 50c |
| 11 | Cu(OTf)2 | 67c |
| 12 | CuBr·SMe2 | 4 |
| 13 | CuNPs/ZY | 99 |
Thiol arylation
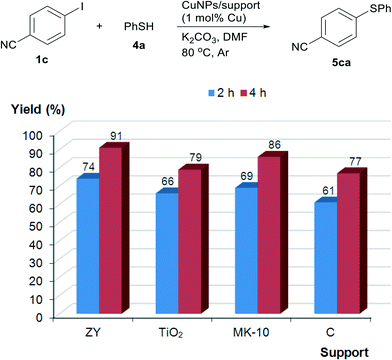
Owing to the paramount importance of thioethers in diverse disciplines, the transition-metal catalysed formation of C–S bonds has attracted a great deal of attention lately.5c,22 We also decided to explore the capability of the supported copper nanoparticles to catalyse the thiophenol arylation; 4-iodobenzonitrile (1c) and thiophenol (4a) were first studied as model substrates. We were pleased to find that, irrespective of the catalyst, the reaction afforded the coupling product in almost quantitative yields when carried out in DMF and K2CO3 as the base for 2 h at 120 °C. The presence of air or absence of solvent had a detrimental effect on the conversion. A lower temperature (80 °C) allowed a better comparison of the activities of the catalysts. Even so, all catalysts manifested quite analogous activity at relatively low copper loadings but, again, CuNPs/ZY was the most active, giving the product in 91% yield after 4 h (Fig. 3). The reactivity of the catalysts followed the order CuNPs/ZY > CuNPs/MK-10 > CuNPs/TiO2 ≥ CuNPs/C.Although 5ca was formed in 94% conversion after 4 h at 100 °C, we decided to conduct the substrate scope at 120 °C in order to maximise the yield using CuNPs/ZY at a ca. 0.7 mol% catalyst loading [as determined from the Cu content (3.0 wt%) and the Cu2O/CuO area from XPS (ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
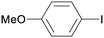
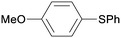
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)] (Table 3). Thiophenol (4a) was successfully coupled with a series of aryl iodides bearing electron-donating and -withdrawing groups as well as halogens. As expected, 4-iodoanisole (1a) reacted more sluggishly giving rise to 5aa in moderate yield, whereas excellent yield was recorded for 4-iodotoluene (1b). The opposite behaviour was noticed for 4-iodobenzonitrile (1c) which furnished the expected diaryl thioether 5ac in only 2 h. The halogenated iodides 1f and 1g reacted chemoselectively towards the activation of the C–I bond, producing the corresponding 4-halogenated thioethers (5fa and 5ga) in near quantitative yields. The presence of electron-donating groups in aryl bromides and chlorides (1l–1j) made them practically unreactive under these reaction conditions. To our surprise, aryl chlorides bearing electron-withdrawing substituents at the 4- or 2-positions (1o–1r) participated in this reaction with high efficiency, giving the corresponding coupling products (5oa–5ra) in high yields after 2 h.
1)] (Table 3). Thiophenol (4a) was successfully coupled with a series of aryl iodides bearing electron-donating and -withdrawing groups as well as halogens. As expected, 4-iodoanisole (1a) reacted more sluggishly giving rise to 5aa in moderate yield, whereas excellent yield was recorded for 4-iodotoluene (1b). The opposite behaviour was noticed for 4-iodobenzonitrile (1c) which furnished the expected diaryl thioether 5ac in only 2 h. The halogenated iodides 1f and 1g reacted chemoselectively towards the activation of the C–I bond, producing the corresponding 4-halogenated thioethers (5fa and 5ga) in near quantitative yields. The presence of electron-donating groups in aryl bromides and chlorides (1l–1j) made them practically unreactive under these reaction conditions. To our surprise, aryl chlorides bearing electron-withdrawing substituents at the 4- or 2-positions (1o–1r) participated in this reaction with high efficiency, giving the corresponding coupling products (5oa–5ra) in high yields after 2 h.
| Aryl halide | t (h) | Product | Yieldb (%) |
|---|---|---|---|
| a Aryl halide (1, 0.25 mmol), thiophenol (4a, 1.5 equiv.), CuNPs/ZY (0.7 mol%) and K2CO3 (0.5 mmol) in DMF (1 mL) at 120 °C under Ar. b 1H NMR yield. | |||
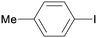
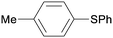
 1a
1a
|
16 |
 5aa
5aa
|
60 |
 1b
1b
|
16 |
 5ba
5ba
|
97 |
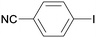 1c
1c
|
2 |
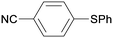 5ca
5ca
|
99 |
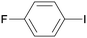 1f
1f
|
8 |
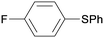 5fa
5fa
|
96 |
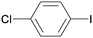 1g
1g
|
8 |
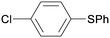 5ga
5ga
|
96 |
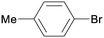 1l
1l
|
16 |
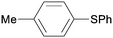 5ba
5ba
|
15 |
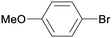 1j
1j
|
16 |
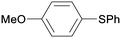 5aa
5aa
|
0 |
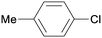 1m
1m
|
16 |
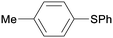 5ba
5ba
|
0 |
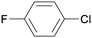 1n
1n
|
16 |
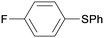 5fa
5fa
|
0 |
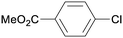 1o
1o
|
2 |
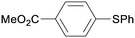 5oa
5oa
|
95 |
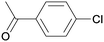 1p
1p
|
2 |
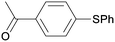 5pa
5pa
|
99 |
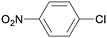 1q
1q
|
2 |
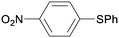 5qa
5qa
|
95 |
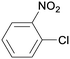 1r
1r
|
2 |
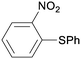 5ra
5ra
|
99 |
The same procedure at a slightly lower temperature (100 °C) was extended to thiols other than thiophenol, including other aromatic (4b–4d), heteroaromatic (4e–4f) and benzylic (4g) thiols; 4-iodobenzonitrile (1c) was selected as a common partner to furnish the expected thioethers in moderate-to-excellent yields in a relatively short reaction time (4 h) (Table 4). Furthermore, thiolation was feasible for all types of aliphatic thiols, i.e., linear-alkyl (4h), branched (4i) and cyclic thiols (4j); a stronger base (KOH) is, by any means, recommended for upgrading the yields. It is noteworthy that 2,2-dimethyl-ethanethiol (4i) reacted quantitatively towards the alkylthio benzonitrile 5ci under milder conditions (70 °C), whereas the corresponding amide (5si) was selectively formed at 120 °C.
| Thiol | Product | Yieldb (%) |
|---|---|---|
| a 4-Iodobenzonitrile (1c, 0.5 mmol), thiol (4, 1.5 equiv.), CuNPs/ZY (0.7 mol%) and K2CO3 (1.0 mmol) in DMF (2 mL) at 100 °C for 4 h under Ar, unless otherwise stated. b Isolated yield. c Reaction using KOH as the base (1.0 mmol) at 120 °C. d Reaction using KOH as the base (1.0 mmol) at 70 °C. | ||
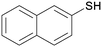 4b
4b
|
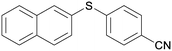 5cb
5cb
|
98 |
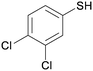 4c
4c
|
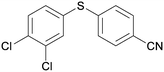 5cc
5cc
|
45 |
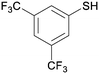 4d
4d
|
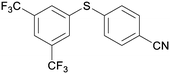 5cd
5cd
|
81 |
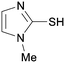 4e
4e
|
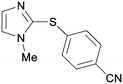 5ce
5ce
|
52 |
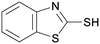 4f
4f
|
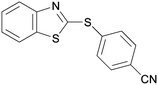 5cf
5cf
|
63 |
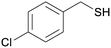 4g
4g
|
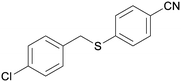 5cg
5cg
|
92 |
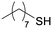 4h
4h
|
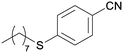 5ch
5ch
|
80c |
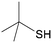 4i
4i
|
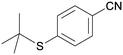 5ci
5ci
|
97d |
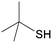 4i
4i
|
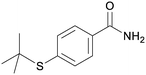 5si
5si
|
60c |
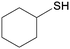 4j
4j
|
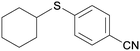 5cj
5cj
|
87c |
Notwithstanding the considerable amount of studies on CuNPs as catalysts for C–S bond formation,7d,23 they are generally applied to the coupling of thiols with aryl iodides. The coupling with aryl chlorides is limited to a few examples,7d,23f,g normally, chloroaromatics bearing electron-withdrawing groups which are coupled with thiophenols.7,23f In other cases, coupling with electron-rich iodides (e.g., 4-iodoanisole) was documented to be troublesome,7,23a whereas very seldom the catalyst was not reusable.23b In our case, the nature of CuNPs/ZY differs from that in the literature examples and leads to a catalytic activity between that of Cu(0)/Cu(I)NPs7d and CuONPs,23f,g albeit the performance with aliphatic thiols is unknown in some cases.7d,23f
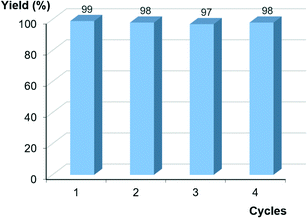
The recycling studies in the coupling of 4-iodobenzonitrile (1c) and thiophenol (4a) followed a pattern resembling that in the Sonogashira reaction, i.e., the catalyst could be reused in four consecutive runs with no apparent decrease in catalytic activity (Fig. 4). The hot filtration test brought forth 0.12% and 0.02% copper leaching after the first and fourth runs, respectively, which are very close to those observed in the Sonogashira reaction and were also catalytically inert.
Apparently, the nanoparticle–support interaction in CuNPs/ZY is independent of the type of reaction implemented, even when they are so different. As has occurred in the Sonogashira reaction, particle agglomeration was not noticeable after reuse (Fig. S5, ESI†).
Recently, in an interesting research, Ananikov et al. have revealed that unsupported copper oxide-catalysed coupling of aryl halides and thiols takes place through leaching from the surface involving the formation of a copper thiolate.24 In contrast with our study, the leached copper species were found to be catalytically active.
The catalytic activity of CuNPs/ZY was compared with that of Cu(0) and an array of commercial Cu(I) and Cu(II) catalysts in the reaction of 4-iodobenzonitrile (1c) and thiophenol (4a) (Table 5). The heterogeneous catalysts, Cu(0), Cu2O and CuO, gave the lowest conversions into the thioether 5ca with the concurrent and abundant formation of the corresponding disulfide (Table 5, entries 1–3). CuCl2 (our copper source to generate the CuNPs) was the best one within the copper halides, though the conversion was only moderate (Table 5, entries 4–7). However, good-to-high conversions were recorded for Cu(OAc)2, CuOTf, Cu(OTf)2 and CuBr·SMe2 (Table 5, entries 9–12). Nonetheless, CuNPs/ZY can be considered the best choice for this reaction because it led to the highest conversion and the catalyst is recyclable (Table 5, entry 13).
| Entry | Catalyst | Conversionb (%) |
|---|---|---|
| a 1c (0.25 mmol), 4a (1.5 equiv.), Cu catalyst (1 mol%) and K2CO3 (0.5 mmol), DMF (1 mL), 100 °C, Ar, 4 h. b Conversion into 5ca determined by GLC based on 1c. | ||
| 1 | Cu(0) | 25 |
| 2 | Cu2O | 23 |
| 3 | CuO | 32 |
| 4 | CuCl | 54 |
| 5 | CuCl2 | 64 |
| 6 | CuBr | 38 |
| 7 | CuI | 50 |
| 8 | CuOAc | 63 |
| 9 | Cu(OAc)2 | 91 |
| 10 | CuOTf | 77 |
| 11 | Cu(OTf)2 | 82 |
| 12 | CuBr·SMe2 | 77 |
| 13 | CuNPs/ZY | 94 |
In general, thiol arylation seems to be less dependent on the support, oxidation state and source of Cu (Fig. 3 and Table 5) when put alongside the Sonogashira reaction (Fig. 1 and Table 2).
Arylation of azoles
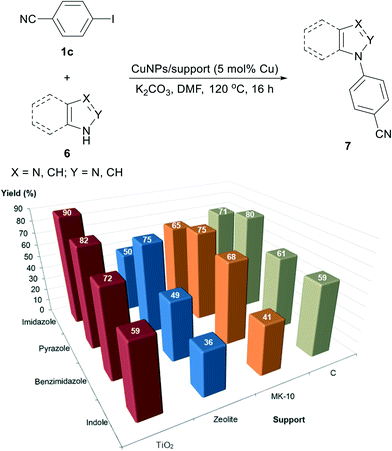
The formation of C(aryl)–N bonds by the coupling of aryl halides and nitrogen nucleophiles has become one of the most studied copper-catalysed reactions in the last ten years.2,25 This is not only a valuable tool for the synthesis of heterocycles25a,c,f but also a transformation applicable to large-scale production in the pharmaceutical industry.3b,25d Although most of the research has been focused on homogeneous catalytic systems,25e heterogeneous copper-catalysed processes, including copper nanoparticles,5a,c have gained more attention in recent times.Recently, the preparation and use of CuNPs/MagSilica in the N-arylation of imidazole has been reported.26 This catalyst successfully catalysed the N-(hetero)arylation of imidazole with (hetero)aryl bromides and iodides but efforts to apply CuNPs/MagSilica to the arylation of other azoles (pyrazole, benzotriazole and indole) were unfruitful. Herein, we have deployed CuNPs on other supports in order to arylate a set of azoles [imidazole (6a), pyrazole (6b), benzimidazole (6c) and indole (6d)] and compare their catalytic activities.
The reactions were implemented using 4-iodobenzonitrile (1c) as a common coupling partner, under equivalent conditions to those for the previous N-arylation of imidazole:26 catalyst (5 mol% Cu), DMF, K2CO3 as the base at 120 °C (instead of 152 °C); the product yields were determined after 16 hours. The reactions proceeded in a selective manner, i.e., the aryl iodide was exclusively converted into the product with no side reactions (Fig. 5). The four catalysts were proven to be active in the N-arylation of all azoles but exhibiting different activities. In contrast to the copper-catalysed Sonogashira reaction and thiol arylation, in this case, CuNPs/TiO2 was the most active catalyst for all the studied azoles. Their general catalytic activity follows the sequence Cu/TiO2 > Cu/C ≈ Cu/MK-10 > Cu/ZY.
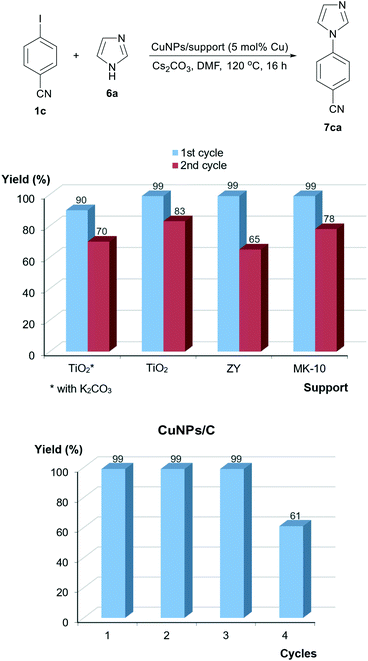
Pyrazole (6b) was found to be the most reactive azole, producing yields in the range of 75–82%, whereas indole was the least reactive one (36–59%). It is noteworthy that CuNPs/TiO2 clearly outmatched the other catalysts in the reaction with imidazole (6a); taking into account that it is mainly composed of Cu2O, the real catalyst loading was ca. 1.6 mol%. Therefore, the nature of the support seems to exert an influence not only on the activity of CuNPs but also on the reactivity of the azoles. Notably, quantitative yields of N-arylated imidazole were reached when employing Cs2CO3 as a base instead of K2CO3, irrespective of the catalyst utilised (Fig. 6).
The arylation of azoles with aryl halides has been effected with copper nanoparticles in different oxidation states [Cu(0)NPs,27a–d CuINPs,27e Cu2ONPs27f,g and CuONPs27h–l]. Nevertheless, some mechanistic studies consider that Cu(I) species are implicated in the first steps of the catalytic cycles.28 This could be a reason whereby CuNPs/TiO2, mainly composed of Cu2O, displayed better catalytic activity than the other catalysts, which are made of mixtures of Cu2O and CuO or of CuO.
An attempt to recycle the most active catalyst (CuNPs/TiO2) in the coupling of imidazole (6a) with 4-iodobenzonitrile (1c) was unsuccessful; a significant decrease in product yield was observed when the catalyst was reused with K2CO3 as the base (Fig. 6). As mentioned before, Cs2CO3 usage increased the product yield in the first cycle, but a decrease after recycling also took place (from 99 to 83%). A similar trend was observed for ZY and MK-10 (65 and 78% in the second run, respectively); the support seems to influence not only the catalytic activity but also the possibility of recycling. It was gratifying, however, to check that CuNPs/C led to a quantitative yield that was preserved in three cycles; a yield decrease was observed only in the fourth cycle (61%) (Fig. 6).
The leaching issue was assessed for CuNPs/TiO2 and CuNPs/C in the reaction of 4-iodobenzonitrile (1c) and imidazole (6a). Using CuNPs/TiO2 as a catalyst, 44% conversion was noted in the first run after 4 h (with catalyst). The catalyst was then removed by hot filtration after this 4 h period and the reaction heated for a further 12 h (without catalyst), giving a conversion of 47% after this total 16 h period. The copper content in the filtrate was determined to be 0.02 wt% of the original amount. In the case of CuNPs/C, the leaching was also marginal in both the second (0.005 wt%) and fourth cycles (0.05 wt%). The negligible, catalytically inactive leaching detected with CuNPs/TiO2 reveals a quite strong metal–support interaction in the catalyst, with no notable change in the latter after reuse (Fig. S5 and S6, ESI†). These facts point to a possible poisoning effect as the main reason for partial deactivation and yield depletion upon recycling CuNPs/TiO2. Metal oxides possess surface acid–base properties, which can facilitate the adsorption and accumulation of heteroatom-containing species. Conversely, CuNPs/C, with the less reactive charcoal surface, must interact more weakly with those species, allowing its efficient reuse in several cycles until certain saturation occurs, with the concomitant yield attenuation.
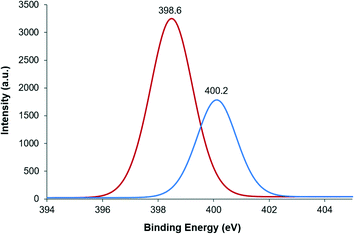
XPS analysis on reused CuNPs/TiO2 at the N 1s level brought into view two peaks at 398.6 and 400.2 eV (Fig. 7). These peaks are consistent with those displayed by fresh CuNPs/TiO2 impregnated with 4-iodobenzonitrile (398.8 and 400.4 eV) (Fig. S7, ESI†) and with that described for imidazole (400.2 eV).29 This reinforces the hypothesis of the starting materials being strongly adsorbed on the TiO2 surface and their poisoning effect upon reuse.
As in the previous coupling reactions, we also compared the catalytic activity of CuNPs/TiO2 with that of the same commercial copper catalysts as above in the arylation of imidazole (6a) with 4-iodobenzonitrile (1c) (Table 6). It is worth noting that Cu2O and CuO, which were rather inactive in both the Sonogashira and thiol arylation reactions, led to conversions of around 75% (Table 6, entries 2 and 3); the behaviour of the Cu(I) and Cu(II) triflates was akin to that of the Cu oxides (Table 6, entries 10 and 11). Still, once more, the nanostructured catalyst showed the highest performance with a quantitative conversion (Table 6, entry 13).
| Entry | Catalyst | Conversionb (%) |
|---|---|---|
| a 1c (0.25 mmol), 6a (1.2 equiv. mmol), catalyst (1.6 mol%) and Cs2CO3 (0.5 mmol), DMF (1 mL), 120 °C, Ar, 16 h. b Conversion into 7ca determined by GLC based on 1c. | ||
| 1 | Cu(0) | 28 |
| 2 | Cu2O | 74 |
| 3 | CuO | 78 |
| 4 | CuCl | 44 |
| 5 | CuCl2 | 46 |
| 6 | CuBr | 18 |
| 7 | CuI | 62 |
| 8 | CuOAc | 25 |
| 9 | Cu(OAc)2 | 52 |
| 10 | CuOTf | 78 |
| 11 | Cu(OTf)2 | 67 |
| 12 | CuBr·SMe2 | 55 |
| 13 | CuNPs/TiO2 | 99 |
Conclusions
We have presented herein a comparative survey on the catalytic activity of four catalysts, comprising copper nanoparticles on different supports (zeolite Y, titania, montmorillonite K-10 and activated carbon), in three types of reactions: the Sonogashira reaction, the arylation of thiols and the arylation of azoles. CuNPs/ZY was the most effective catalyst for the Sonogashira reaction (4 mol% catalyst, K2CO3, DMF, 120 °C), being applicable to aryl iodides bearing either electron-donating or -withdrawing groups; aryl bromides reacted more sluggishly. The same catalyst at a lower loading (0.7 mol%) allowed the coupling of thiophenol with aryl iodides of different electronic character but also with electron-poor aryl chlorides in near quantitative yields. Moreover, alkyl thiols were successfully coupled when KOH was used as the base instead of K2CO3. As regards the azole arylation, CuNPs/TiO2 (1.6 mol%) achieved the highest conversion in the coupling of 4-iodobenzonitrile with imidazole, pyrazole, benzimidazole and indole, using K2CO3 as the base. Anyhow, all the catalysts reached quantitative yields when K2CO3 was changed to Cs2CO3 as the base.This comparative study has been extended to a collection of twelve commercial copper catalysts; only CuOTf and Cu(OTf)2 maintained a moderate-to-good activity in the three reactions examined (50–82% conversion). The rest of the catalysts failed in the Sonogashira reaction (<9% conversion), whereas CuBr·SMe2 and Cu(OAc)2 in the thiol arylation, and Cu2O and CuO in the azole arylation gave comparable results to those attained with the copper(I) and (II) triflates. At any rate, in general, the nanoparticulate supported catalysts are markedly superior to the commercial catalysts in terms of catalytic activity and reusability; they can be reused in four (Sonogashira and thiol arylation) and three (azole arylation) cycles with no loss of activity. The negative filtration test and insignificant leaching lend weight to the argument that the catalysis is heterogeneous, taking place at the nanoparticle surface; the possibility of leached Cu from the CuNPs which get into a homogeneous catalytic cycle can, practically, be ruled out. Hence, taking into account that the catalysts are easily prepared, the protocols introduced in this report are an attractive alternative to the utilisation of the more expensive palladium catalysts and commercial (non-reusable) copper catalysts.
Experimental
General procedure for the preparation of the catalysts
All the supported copper catalysts handled in this work were prepared by adding the support (titania,8a zeolite Y,8b activated carbon14b or montmorillonite K-10) to a newly prepared suspension of the CuNPs readily generated, in turn, by the chemical reduction of copper(II) chloride with lithium metal and a catalytic amount of 4,4′-di-tert-butylbiphenyl (DTBB) as an electron carrier. In a general procedure: anhydrous copper(II) chloride (134 mg, 1 mmol) was added to a suspension of lithium (14 mg, 2 mmol) and DTBB (27 mg, 0.1 mmol) in THF (2 mL) at room temperature under an argon atmosphere. The reaction mixture, which was initially dark blue, rapidly changed to black, indicating that a suspension of copper nanoparticles was formed. This suspension was diluted with THF (18 mL) followed by the addition of the support (1.28 g). The resulting mixture was stirred for 1 h at room temperature, filtered, and the solid was successively washed with water (20 mL), THF (20 mL), and dried under vacuum. The supported catalysts were not subjected to any other treatment prior to use.General procedure for the cross coupling of aryl iodides (1) with arylacetylenes (2) catalysed by CuNPs/ZY (Table 1)
The aryl halide (1, 0.25 mmol), arylacetylene (2, 0.375 mmol, 1.5 equiv.), CuNPs/ZY (26.7 mg, ca. 4 mol%), K2CO3 (69 mg, 0.5 mmol) and DMF (1 mL) were added to a reactor tube. The mixture was warmed to 120 °C under Ar and stirred for the specified time in Table 1. The reaction crude was diluted with EtOAc (3 mL) and filtered through a pad with Celite, followed by extraction of the filtrate with water (3 × 3 mL) to remove the DMF, washing with brine (4 mL) and drying with anhydrous MgSO4. The resulting organic phase was subjected to solvent evaporation under vacuum and to 1H NMR analysis (mesitylene as the internal standard) for products 3aa–3ka or to purification by column chromatography (silica gel, hexane/EtOAc) for products 3cb–3cd.General procedure for the cross coupling of aryl halides (1) with thiophenol (4a) catalysed by CuNPs/ZY (Table 3)
The aryl halide (1, 0.25 mmol), thiophenol (4a, 38 μL, 0.375 mmol, 1.5 equiv.), CuNPs/ZY (5.3 mg, 0.7 mol%), K2CO3 (69 mg, 0.5 mmol) and DMF (1 mL) were added to a reactor tube. The mixture was warmed to 120 °C under Ar and stirred for the specified time in Table 3. The reaction crude was diluted with EtOAc (3 mL) and filtered through a pad with Celite, followed by extraction of the filtrate with water (3 × 3 mL) to remove the DMF, washing with brine (4 mL) and drying with anhydrous MgSO4. The resulting organic phase was subjected to solvent evaporation under vacuum and 1H NMR analysis (mesitylene as the internal standard).General procedure for the cross coupling of 4-iodobenzonitrile (1c) with thiols (4) catalysed by CuNPs/ZY (Table 4)
4-Iodobenzonitrile (1c, 114.5 mg, 0.5 mmol), the thiol (4, 0.75 mmol, 1.5 equiv.), CuNPs/ZY (10.6 mg, 0.7 mol%), K2CO3 (138 mg, 1.0 mmol) or KOH (1.0 mmol) and DMF (2 mL) were added to a reactor tube. The mixture was warmed to 70 or 100 °C under Ar and stirred for 4 h. The reaction crude was diluted with EtOAc (6 mL) and filtered through a pad with Celite, followed by extraction of the filtrate with water (3 × 6 mL) to remove the DMF and washing with brine (8 mL). The resulting organic phase was subjected to solvent evaporation under vacuum and purification by column chromatography (silica gel, hexane/EtOAc).General procedure for the cross coupling of 4-iodobenzonitrile (1c) with azoles (6)
4-Iodobenzonitrile (1c, 57.3 mg, 0.25 mmol), the corresponding azole (6, 0.3 mmol, 1.2 equiv.), the CuNP catalyst (5 mol% Cu), K2CO3 (69 mg, 0.5 mmol) and DMF (1 mL) were added to a reactor tube. The mixture was warmed to 120 °C under Ar and stirred for 16 h. The reaction crude was diluted with EtOAc (3 mL) and filtered through a pad with Celite, followed by extraction of the filtrate with water (3 × 3 mL) to remove the DMF, washing with brine (4 mL) and drying with anhydrous MgSO4. The resulting organic phase was subjected to solvent evaporation under vacuum and 1H NMR analysis (mesitylene as the internal standard).Conflicts of interest
There are no conflicts to declare.Acknowledgements
I. P. Beletskaya thanks the Russian Science Foundation (RSF, grant no. 14-23-00186 P) and A. Yu. Mitrofanov thanks the Russian Foundation for Basic Research (grant no. 16-33-60207) for their financial support. This work was also generously supported by the Spanish Ministerio de Economía y Competitividad (MINECO; grant no. CTQ-2015-66624-P) and the Institute of Organic Synthesis (ISO). I. M.-G. thanks the ISO and the Vicerrectorado de Investigación y Transferencia del Conocimiento of the Universidad de Alicante for pre-doctoral grants (no. UAFPU2016-034).Notes and references
- Reviews and monographs:
(a) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337–2364 CrossRef CAS
; (b) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954–6971 CrossRef CAS PubMed
; (c) L. Penn and D. Gelman, in The Chemistry of Organocopper Compounds, ed. Z. Rappoport and I. Marek, John Wiley & Sons, Hoboken (NJ), 2009, ch. 18, pp. 881–990 Search PubMed
; (d) H. Rao and H. Fu, Synlett, 2011, 745–769 CAS
; (e) I. P. Beletskaya and A. V. Cheprakov, Organometallics, 2012, 31, 7753–7808 CrossRef CAS
; (f) Copper-Mediated Cross-Coupling Reactions, ed. G. Evano and N. Blanchard, John Wiley & Sons, Hoboken (NJ), 2014 Search PubMed
; (g) C. Maaliki, E. Thiery and J. Thibonnet, Eur. J. Org. Chem., 2017, 209–228 CrossRef CAS
.
-
(a)
Y. Jian and D. Ma, in Catalysis Without Precious Metals, ed. R. M. Bullock, Wiley-VCH, Weinheim, 2010, ch. 9, pp. 213–233 Search PubMed
; (b) C. Sambiagio, S. P. Marsden, A. J. Blacker and P. C. McGowan, Chem. Soc. Rev., 2014, 43, 3525–3550 RSC
.
-
(a) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054–3131 CrossRef CAS PubMed
; (b) J. Yin, in Applications of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective, John Wiley & Sons, Hoboken (NJ), 1st edn, 2012, ch. 3, pp. 97–163 Search PubMed
.
-
(a)
M. L. Kantam, C. V. Reddy, P. Srinivas and S. Bhargava, in Topics in Organometallic Chemistry, ed. M. Taillefer and D. Ma, Springer, Heidelberg, 2013, vol. 46, pp. 119–171 Search PubMed
; (b) Z. Wang, C. Wan and Y. Wang, in Copper-Mediated Cross-Coupling Reactions, ed. G. Evano and N. Blanchard, John Wiley & Sons, Hoboken (NJ), 2014, ch. 20, pp. 745–784 Search PubMed
.
- Reviews:
(a) B. C. Ranu, R. Dey, T. Chatterjee and S. Ahammed, ChemSusChem, 2012, 5, 22–44 CrossRef CAS PubMed
; (b) R. Chinchilla and C. Nájera, in Nanocatalysis: Synthesis and Applications, ed. V. Polshettiwar and T. Asefa, John Wiley & Sons, Hoboken (NJ), 1st edn, 2013, ch. 4, pp. 89–132 Search PubMed
; (c) B. C. Ranu, D. Saha, D. Kundu and N. Mukherjee, in Nanocatalysis: Synthesis and Applications, ed. V. Polshettiwar and T. Asefa, John Wiley & Sons, Hoboken (NJ), 1st edn, 2013, ch. 6, pp. 189–220 Search PubMed
; (d) M. B. Gawande, A. Goswami, F.-X. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril and R. S. Varma, Chem. Rev., 2016, 116, 3722–3811 CrossRef CAS PubMed
; (e) L. Shiri, A. Ghorbani-Choghamarani and M. Kazemi, Aust. J. Chem., 2016, 69, 585–600 CrossRef CAS
.
- For an example of inseparable CuNPs, see:
(a) P. Abdulkin, Y. Moglie, B. R. Knappett, D. A. Jefferson, M. Yus, F. Alonso and A. E. H. Wheatley, Nanoscale, 2013, 5, 342–350 RSC
Reviews: (b) J. Fan and Y. Gao, J. Exp. Nanosci., 2006, 1, 457–475 CrossRef CAS
; (c) J. Sun and X. Bao, Chem. – Eur. J., 2008, 14, 7478–7488 CrossRef CAS PubMed
; (d) R. J. White, R. Luque, V. L. Budarin, J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 2009, 38, 481–494 RSC
; (e) J. M. Campelo, D. Luna, R. Luque, J. M. Marinas and A. A. Romero, ChemSusChem, 2009, 2, 18–45 CrossRef CAS PubMed
; (f) P. Munnik, P. E. de Jongh and K. P. de Jong, Chem. Rev., 2015, 115, 6687–6718 CrossRef CAS PubMed
.
- See, for instance: (C–C)
(a) R. Arundhathi, D. Damodara, K. V. Mohan, M. Lakshmi Kantam and P. R. Likhar, Adv. Synth. Catal., 2013, 355, 751–756 CrossRef CAS
; (C–N) (b) A. R. Hajipour, F. Dordahan, F. Rafiee and M. Mahdavi, Appl. Organomet. Chem., 2014, 28, 809–813 CrossRef CAS
; (c) P. L. Reddy, R. Arundhathi and D. S. Rawat, RSC Adv., 2015, 5, 92121–92127 RSC
; (C–S) (d) S. Findy, A. el Kadib, M. Lahcini and H. García, ChemCatChem, 2015, 7, 3307–3315 CrossRef
; (C–O) (e) P. Puthiaraj and W.-S. Ahn, Catal. Sci. Technol., 2016, 6, 1701–1709 RSC
; (C–P) (f) V. Gutiérrez, E. Mascaró, F. Alonso, Y. Moglie and G. Radivoy, RSC Adv., 2015, 5, 65739–65744 RSC
.
-
(a) F. Alonso, T. Melkonian, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2011, 2524–2530 CrossRef CAS
; (b) F. Alonso, A. Arroyo, I. Martín-García and Y. Moglie, Adv. Synth. Catal., 2015, 357, 3549–3561 CrossRef CAS
.
- Reviews:
(a) T. Jin, M. Yan and Y. Yamamoto, ChemCatChem, 2012, 4, 1217–1229 CrossRef CAS
; (b) F. Alonso, Y. Moglie and G. Radivoy, Acc. Chem. Res., 2015, 48, 2516–2528 CrossRef CAS PubMed
; see, also (c) M. J. Albaladejo, F. Alonso and M. J. González-Soria, ACS Catal., 2015, 5, 3446–3456 CrossRef CAS
.
-
Carbon Materials for Catalysis, ed. P. Serp and J. L. Figueiredo, John Wiley & Sons, Hoboken (NJ), 2009 Search PubMed
.
- Reviews:
(a) X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed
; (b) A. Primo, A. Corma and H. García, Phys. Chem. Chem. Phys., 2011, 13, 886–910 RSC
; (c) Z. W. Seh, S. Liu and M.-Y. Han, Chem. – Asian J., 2012, 7, 2174–2184 CrossRef CAS PubMed
; (d) V. Hulea and E. Dumitriu, in Nanomaterials in Catalysis, ed. P. Serp and K. Philippot, Wiley-VCH, Weinheim, 1st edn, 2013, ch. 10, pp. 375–413 Search PubMed
; (e) For a special issue on TiO2 nanomaterials, see: Chem. Rev., 2014, 11419 Search PubMed.
- Review:
(a) D. K. Dutta, B. J. Borah and P. P. Sarmah, Catal. Rev.: Sci. Eng., 2015, 57, 257–305 CrossRef CAS
; (b) for a recent article about CuNPs on montmorillonite, see: W. Lang, Q. Yang, X. Song, M. Yin and L. Zhou, RSC Adv., 2017, 7, 13754–13759 RSC
.
- Reviews and monographs:
(a)
Zeolites and Catalysis, ed. J. Cejka, A. Corma and S. Zones, Wiley-VCH, Weinheim, 2010 Search PubMed
; (b) B. Louis, G. Laugel, P. Pale and M. M. Pereira, ChemCatChem, 2011, 3, 1263–1272 CrossRef CAS
; (c) M. Moliner, C. Martínez and A. Corma, Angew. Chem., Int. Ed., 2015, 54, 3560–3579 CrossRef CAS PubMed
; (d) M. P. Singh, G. S. Baghel, S. J. J. Titinchi and H. S. Abbo, in Advanced Catalytic Materials, ed. A. Tiwari and S. Titinchi, Scrivener Publishing LLC, 2015, ch. 11, pp. 385–410 Search PubMed
; (e) For a special issue on the chemistry of zeolites, see: Chem. Soc. Rev., 2015, 4420 Search PubMed.
- See, for instance:
(a) F. Alonso, P. Riente, F. Rodríguez-Reinoso, J. Ruiz-Martínez, A. Sepúlveda-Escribano and M. Yus, ChemCatChem, 2009, 1, 75–77 CrossRef CAS
; (b) F. Alonso, Y. Moglie, G. Radivoy and M. Yus, J. Org. Chem., 2013, 78, 5031–5037 CrossRef CAS PubMed
.
- See, for instance:
(a) F. Alonso, R. Buitrago, Y. Moglie, A. Sepúlveda-Escribano and M. Yus, Organometallics, 2012, 31, 2336–2342 CrossRef CAS
; (b) F. Alonso, Y. Moglie, L. Pastor-Pérez and A. Sepúlveda-Escribano, ChemCatChem, 2014, 6, 857–865 CrossRef CAS
.
-
(a) F. Alonso, J. J. Calvino, I. Osante and M. Yus, J. Exp. Nanosci., 2006, 1, 419–433 CrossRef CAS
; (b) F. Alonso and M. Yus, Pure Appl. Chem., 2008, 80, 1005–1012 CrossRef CAS
.
- Reviews:
(a) R. Chinchilla and C. Najera, Chem. Rev., 2007, 40, 874–922 CrossRef PubMed
; (b) H. Doucet and J.-C. Hierso, Angew. Chem., Int. Ed., 2007, 46, 834–871 CrossRef CAS PubMed
; (c) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS PubMed
; (d) M. D. Heravi and S. Sadjadi, Tetrahedron, 2009, 65, 7761–7775 CrossRef CAS
; (e) M. Pal, Synlett, 2009, 2896–2912 CrossRef CAS
; (f) R. Chinchilla and C. Najera, Chem. Soc. Rev., 2011, 40, 5084–5121 RSC
; (g) M. Bakherad, Appl. Organomet. Chem., 2013, 27, 125–140 CrossRef CAS
; (h) D. Wang and S. Gao, Org. Chem. Front., 2014, 1, 556–566 RSC
; (i) M. Karak, L. C. A. Barbosa and G. C. Hargaden, RSC Adv., 2014, 4, 53442–53466 RSC
.
- Reviews:
(a) A. M. Thomas, A. Sujatha and G. Anilkumar, RSC Adv., 2014, 4, 21688–21698 RSC
; (b) R.-J. Song and J.-H. Li, in Copper-Mediated Cross-Coupling Reactions, ed. G. Evano and N. Blanchard, John Wiley & Sons, Hoboken (NJ), 2014, ch. 11, pp. 405–421 Search PubMed
.
-
(a) M. B. Thathagar, J. Beckers and G. Rothenberg, Green Chem., 2004, 6, 215–218 RSC
; (b) Y. Yuan, H. Zhu, D. Zhao and L. Zhang, Synthesis, 2011, 1792–1798 CrossRef CAS
; (c) A. Biffis, E. Scattolin, N. Ravasio and F. Zaccheria, Tetrahedron Lett., 2007, 48, 8761–8764 CrossRef CAS
.
-
(a) M. Planellas, Y. Moglie, F. Alonso, M. Yus, R. Pleixats and A. Shafir, Eur. J. Org. Chem., 2014, 3001–3008 CrossRef CAS
; (b) A. S. Camacho, I. Martín-García, C. Contreras-Celedón, L. Chacón-García and F. Alonso, Catal. Sci. Technol., 2017, 7, 2262–2273 RSC
.
- For a perspective on alkyne homocoupling, see: F. Alonso and M. Yus, ACS Catal., 2012, 2, 1441–1451 CrossRef CAS
.
- Reviews:
(a) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596–1636 CrossRef CAS PubMed
; (b) C. C. Eichman and J. P. Stambuli, Molecules, 2011, 16, 590–608 CrossRef CAS PubMed
; (c) C.-F. Lee, Y.-C. Liu and S. S. Badsara, Chem. – Asian J., 2014, 9, 706–722 CrossRef CAS PubMed
; (d) A. Sujatha, A. M. Thomas, A. P. Amrutha and G. Anilkumar, ARKIVOC, 2015,(i), 1–28 Search PubMed
.
-
(a) L. Rout, T. K. Sen and T. Punniyamurthy, Angew. Chem., Int. Ed., 2007, 46, 5583–5586 CrossRef CAS PubMed
; (b) B. C. Ranu, A. Saha and R. Jana, Adv. Synth. Catal., 2007, 349, 2690–2696 CrossRef CAS
; (c) C. Gonzalez-Arellano, R. Luque and D. J. Macquarrie, Chem. Commun., 2009, 1410–1412 RSC
; (d) R. S. Schwab, D. Singh, E. E. Alberto, P. Piquini, O. E. D. Rodrigues and A. L. Braga, Catal. Sci. Technol., 2011, 1, 569–573 RSC
; (e) P. Veerakumar, M. Velayudham, K.-L. Lu and S. Rajagopal, Catal. Sci. Technol., 2011, 1, 1512–1525 RSC
; (f) S. G. Babu and R. Karvembu, Tetrahedron Lett., 2013, 54, 1677–1680 CrossRef CAS
; (g) A. Kamal, V. Srinivasulu, J. N. S. R. C. Murty, N. Shankaraiah, N. Nagesh, T. Srinivasa Reddy and A. V. Subba Rao, Adv. Synth. Catal., 2013, 355, 2297–2307 CrossRef CAS
.
- Y. S. Panova, A. S. Kashin, M. G. Vorobev, E. S. Degtyareva and V. P. Ananikov, ACS Catal., 2016, 6, 3637–3643 CrossRef CAS
.
- Reviews:
(a) G. Evano, M. Toumi and A. Coste, Chem. Commun., 2009, 4166–4175 RSC
; (b) Y. Aubin, C. Fischmeister, C. M. Thomas and J.-L. Renaud, Chem. Soc. Rev., 2010, 39, 4130–4145 RSC
; (c) J. E. R. Sadig and M. C. Willis, Synthesis, 2011, 1–22 CAS
; (d) D. Ma and Y. Jiang, Chimia, 2011, 65, 914–918 CrossRef CAS PubMed
; (e) F. Monnier and M. Taillefer, in Amination and Formation of sp2 C-N Bonds, Top. Organomet. Chem., ed. M. Taillefer and D. Ma, Springer, Heidelberg, 2013, vol. 46, pp. 173–204 Search PubMed
; (f) J. Bariwalab and E. Van der Eycken, Chem. Soc. Rev., 2013, 42, 9283–9303 RSC
; (g) K. Okano, H. Tohuyama and T. Fukuyama, Chem. Commun., 2014, 50, 13650–13663 RSC
.
- F. Nador, M. A. Volpe, F. Alonso and G. Radivoy, Tetrahedron, 2014, 70, 6082–6087 CrossRef CAS
.
- Cu(O)NPs:
(a) M. Kidwai, N. Kumar Mishra, S. Bhardwaj, A. Jahan, A. Kumar and S. Mozumdar, ChemCatChem, 2010, 2, 1312–1317 CrossRef CAS
; (b) Z. Huang, F. Li, B. Chen, F. Xue, G. Chen and G. Yuan, Appl. Catal., A, 2011, 403, 104–111 CrossRef CAS
; (c) G. Pai and P. Chattopadhyay, Tetrahedron Lett., 2014, 55, 941–944 CrossRef CAS
; (d) P. Linga Reddy, R. Arundhathi and D. S. Rawat, RSC Adv., 2015, 5, 92121–92127 RSC
; CuINPs: (e) B. Sreddhar, R. Arundhathi, P. Linga Reddy and M. Lakshmi Kantam, J. Org. Chem., 2009, 74, 7951–7954 CrossRef PubMed
; Cu2ONPs: (f) S. U. Son, I. K. Park, J. Park and T. Hyeon, Chem. Commun., 2004, 778–779 RSC
; (g) B.-X. Tang, S.-M. Guo, M.-B. Zhang and J.-H. Li, Synthesis, 2008, 1707–1716 CAS
; CuONPs (h) L. Rout, S. Jammi and T. Punniyamurthy, Org. Lett., 2007, 9, 3397–3399 CrossRef CAS PubMed
; (i) M. Lakshmi Kantam, J. Yadav, S. Laha, B. Sreddhar and S. Jha, Adv. Synth. Catal., 2007, 349, 1938–1942 CrossRef
; (j) S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971–1976 CrossRef CAS PubMed
; (k) S. Ganesh Babu and R. Karembu, Ind. Eng. Chem. Res., 2011, 50, 9594–9600 CrossRef CAS
; (l) M. Halder, Md. M. Islam, Z. Ansari, S. Ahammed, K. Sen and Sk. M. Islam, ACS Sustainable Chem. Eng., 2017, 5, 648–657 CrossRef CAS
.
- G. Franc and A. Jutand, Dalton Trans., 2010, 39, 7873–7875 RSC
, and the references cited therein.
- A. Singh, K. R. Ansari, A. Kumar, W. Liu, C. Songsong and Y. Lin, J. Alloys Compd., 2017, 712, 121–123 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Catalyst characterisation, experimental procedures, spectroscopic data and NMR spectra of the new compounds. See DOI: 10.1039/c7cy01343d |
| This journal is © The Royal Society of Chemistry 2017 |