Development of basicity in mesoporous silicas and metallosilicates†
Abstract
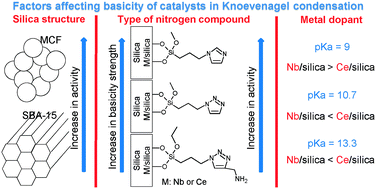
We report herein an experimental study on the development of basicity on mesoporous silicas and metallosilicates (Nb- and Ce-) on SBA-15 and MCF porous structures. Our results demonstrated that the role of the catalyst structure (hexagonally ordered mesoporous SBA-15 vs. mesoporous cellular foam MCF), the effect of the amount and nature of metal in the supports (different acidic/redox properties), and the effect of the type of nitrogen-containing organic modifier (imidazole, triazole and aminotriazole) play an important role on the activity and selectivity in Knoevenagel condensation, which is a well-known probe reaction to determine the basicity of inorganic solids. We demonstrate that niobium Lewis acidity is determinant for the Knoevenagel condensation and, also, redox properties of cerium play an important role in the basicity. In fact, acid–base cooperation plays a positive role in promoting the Knoevenagel condensation via the ion-pair mechanism. These mesoporous silicas and metallosilicates are interesting catalysts for production of fine chemicals due to their thermal stability and controllable acid–base catalytic properties.
- This article is part of the themed collections: 2017 Catalysis Science & Technology HOT Articles and Catalytic reactivity of surfaces: in recognition of François Gault


 Please wait while we load your content...
Please wait while we load your content...