Effect of zeolite confinement on the conversion of 1-butanol to butene isomers: mechanistic insights from DFT based microkinetic modelling†
Abstract
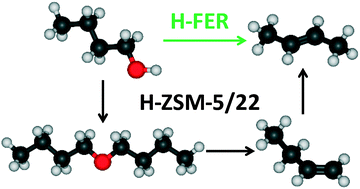
Ab initio based microkinetic modelling of 1-butanol dehydration to butene isomers is used to obtain mechanistic insights into the effect of a zeolite framework. A detailed microkinetic model including double bond isomerization, skeletal isomerization and mechanisms for the direct formation of 2t-butene from 1-butanol dimer and di-1-butyl ether (DBE) is considered for the dehydration in H-ZSM-5, H-ZSM-22 and H-FER. H-FER favors the production of 2t-butene and H-ZSM-22 achieves thermodynamic equilibrium composition for linear butenes even at low conversion levels, while H-ZSM-5 maximizes 1-butene selectivity. Significant differences are observed in the reaction mechanism leading to formation of 2t-butene. For H-ZSM-5 and H-ZSM-22, the formation of 2-butenes occurs via double bond isomerization of 1-butene produced from butanol dehydration. For the double bond isomerization of 1-butene to 2t-butene, both concerted and 2-butoxide mediated stepwise mechanisms contribute significantly in H-ZSM-5, while only the concerted mechanism is operative in H-ZSM-22. On the other hand, for H-FER, 2t-butene is mainly produced from the butanol dimer via an E1 elimination accompanied by a 1,2-hydride shift. This in turn can be attributed to an increase in enthalpic stabilization of the E1 elimination transition state for the direct formation of 2t-butene from 1-butanol dimer when moving from H-ZSM-5 to H-FER. Isobutene formation is not observed in all three zeolites at the investigated temperature range of 450–500 K.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...