State-to-state mode specificity in H + DOH(νOH = 1) → HD + OH(ν2 = 0) reaction: vibrational non-adiabaticity or local-mode excitation?†
Bin
Zhao‡
 *a,
Zhigang
Sun
b and
Hua
Guo
*a,
Zhigang
Sun
b and
Hua
Guo
 a
a
aDepartment of Chemistry and Chemical Biology, University of New Mexico, Albuquerque, New Mexico 87131, USA. E-mail: bzhao@unm.edu
bCenter for Theoretical and Computational Chemistry, and State Key Laboratory of Molecular Reaction Dynamics, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
First published on 24th November 2017
Abstract
It is well established that chemical reactions often involve only a small number of atoms near the reaction site, and the remainder of the reactant molecules are mostly spectators. It is thus of great importance to understand the role played by the active as well as spectator modes in chemical dynamics. In this work, we examine in great detail the influence of reactant modes on the reactivity and product state distribution, using a four-atom prototypical reaction as the example. State-of-the-art full-dimensional state-to-state quantum dynamics reveal a startling observation in which the DOH(νOH = 1) molecule reacts with a H atom to produce a vibrationless OH product. This is surprising because OH is considered as a spectator in this reaction and its internal energy should be sequestered throughout the reaction. By careful analysis within the local-mode regime, we demonstrate that the surprising reactivity is not due to vibrational non-adiabaticity during the reaction. Rather, it can be attributed to a small OD excited local-mode component in the reactant wavefunction. The quantum state-resolved dissection of this prototype reaction helps to advance our understanding of larger reactive systems.
Introduction
Recently, there has been increasing attention paid to quantum dynamical studies of bimolecular reactions beyond the atom–diatom paradigm that has dominated the field of reaction dynamics for a long time.1–4 Reactions with polyatomic reactants are of course more challenging, but also provide a much wider spectrum of interesting phenomena, which have attracted strong experimental interest.5–7 Taking the example of mode specificity, which is of great importance in reaction dynamics,8 a polyatomic molecule with 3N – 6 vibrational modes (N being the number of atoms in the molecule) presents a much richer variety of possibilities, especially when comparing with the single vibrational mode in a diatom. Quantum dynamical studies of such reactions at the state-to-state level is extremely challenging, but much progress has recently been made thanks to the availability of high-quality reactive potential energy surfaces (PESs)9–11 and advances in quantum reactive scattering algorithms.12Our ability to unravel the state-to-state quantum dynamics is not only important for specific prototypical systems, but may also lead to general principles that can be applied to larger systems that are not currently amenable to quantum treatments. It is well established that chemical reactions often occur locally, involving only a small number of active modes. The other modes do not participate chemically. It is thus vital to understand the roles of the active as well as spectator modes in chemical dynamics. In this work, we focus on an atom–triatom system, which is a representative of four-atom reactions.13 We examine by detailed analysis of the state-to-state quantum dynamics of this system the influence of various reactant modes on reactivity and product energy disposal.
The specific isotopologue of the hydrogen abstraction reaction of water by a hydrogen atom is given below:
| H + DOH → HD + OH, | (R1) |
| H + DOH → H2 + OD. | (R1′) |
The mode specificity and bond selectivity of the reaction has also been thoroughly investigated,33,34,44,46,48,50,53 which highlights the dominant role played by the transition state.54,55 It is now well established that the mode specificity can be readily understood in terms of the coupling of the reactant vibrational normal modes with the reaction coordinate at the transition state. This coupling can be readily mapped out by projecting the reactant normal mode vectors on to the reaction coordinate vector, as suggested by the Sudden Vector Projection (SVP) model.56 Very recently, the local-mode nature of the reaction has been addressed in several full-dimensional quantum dynamical studies,50,57 which elucidated the mode specificity in this reaction.
These theoretical studies have greatly advanced our understanding of this prototypical reactive system. It is now commonly accepted that the non-reacting OH mode serves as a spectator mode,14,16,50 which is nearly orthogonal to the reaction coordinate at the transition state.58 As a result, exciting this mode has almost no effect on the reactivity for (R1). (Note that DOH, with the excited OH mode, proceeds largely via (R1′), in which the OH mode is almost perfectly aligned with the corresponding reaction coordinate,58 but that is not the focus of this work.) In addition, energy deposited in the reactant OH mode is expected to stay in the mode throughout the reaction (R1), resulting in vibrationally excited OH. However, as shown below based on state-of-the-art full-dimensional state-to-state quantum calculations, a small amount of the OH product produced from reaction between the OH excited DOH and H atom is found in its ground vibrational state, especially at low collision energies. This surprising result might have two possible explanations. One is vibrational non-adiabaticity, which helps to transfer energy in the excited OH mode to other modes during the reaction, giving rise to the OH(ν2 = 0) product. An alternative, and correct reason as we demonstrate here, is that the “de-excitation” of OH is caused by a minor local-mode component of the excited OD mode in the initial reactant wavefunction, in which the OH local-mode is not excited. Indeed, the localization of the OH and OD vibrations in the excited vibrational eigenstates of the DOH molecule is not complete, which leads to the surprising reactivity. As a result, the mode mixing is already present in the initial state, rather than during the reaction. The results and analysis help to shed light on the behavior of spectator modes in polyatomic reactions.
Theory
Results presented here were obtained using a reactant-coordinate based (RCB) method for state-to-state quantum reactive scattering of four-atom systems. Since this method has been discussed extensively in a recent publication,53 no detail is given here. Briefly, the initial wave packet is launched in the reactant asymptote and propagated in time using an exact kinetic energy operator in the A + BCD reactant Jacobi coordinates, and the S-matrix elements were computed in the product channel via a series of transformations of the six-dimensional time-dependent wave packet. Some calculations were also performed with the potential averaged five-dimensional (PA5D) approximation with the non-reacting OH bond excluded from the dynamical calculations.26 All partial waves were included. The neural network potential energy surface (PES) of the H3O system developed by Chen et al.25 was used. Parameters of the calculation are listed in Table S-I in the ESI.†Results
A. Vibrational mode specificity in total reactivity
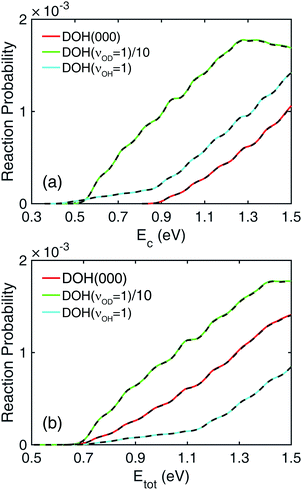
In Fig. 1, total reaction probabilities (Jtot = 0) of the H + DOH → HD + OH reaction are shown with the DOH reactant in three different vibrational states, labeled by (νOD, νb, νOH), which denote the quantum numbers of the OD stretching, bending, and OH stretching modes, respectively. To make different vibrational states of the DOH reactant more explicit, the (100) and (001) states are denoted below as DOH(νOD = 1) and DOH(νOH = 1), respectively. In panel (a), the reaction probabilities are plotted with respect to collision energy. The reaction probability from DOH(νOD = 1) is much larger than the ones from the other two modes by a factor of more than 20. This indicates that the energy deposited in the OD stretching mode is facilely utilized to promote the reaction, which is consistent with the active nature of this mode predicted by the SVP model.58 On the other hand, it is quite interesting to note the unexpected behavior of the reaction probability from DOH(νOH = 1). If the OH bond can truly be considered as a spectator, the total reaction probability from DOH(νOH = 1) should be similar to the one from DOH(000) as the reactivity should not be affected by the vibrational excitation of the non-reacting OH bond. Surprisingly, the total reaction probability from DOH(νOH = 1) is substantially higher than the DOH(000) one and has a lower energy threshold. This behavior was also seen in recent work of Fu and Zhang on the same PES,46 but without an explanation. Specifically, the reaction threshold is lowered to about 0.45 eV, which corresponds to the excitation energy of the DOH(νOH = 1) mode. It thus appears that the energy in the excited OH mode can somehow be used to promote the reaction, despite the fact that it is a spectator. Another distinct feature, regarding the energy dependence of the reaction probability from DOH(νOH = 1), are the apparently two different slopes in the probability with a kink near the threshold energy of the DOH(000) state at around 0.85 eV, as if there are more than one channels that contribute.Before going to the analysis at the state-to-state level, it is worthwhile to plot the same total reaction probabilities with respect to total energy, namely the sum of collision and internal energies. Shown in panel (b) of Fig. 1 where the energy zero is set at the classical minimum energy in the H + DOH asymptote, it is interesting to note the reaction probabilities of the three modes all have roughly the same threshold in total energy, implying the comparable ability of translational energy and stretching vibration in initiating the reaction. It should be noted here that the DOH(νOH = 1) level has roughly 0.1 eV higher internal energy than the DOH(νOD = 1) level. To have the same threshold in total energy as for the DOH(νOH = 1) level, an extra of 0.1 eV translational energy is required for the DOH(νOD = 1) level.
B. Vibrational mode specificity in product state distributions
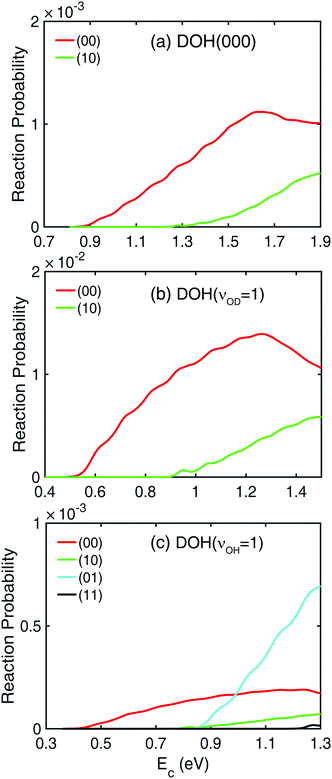
In Fig. 1, Jtot = 0 reaction probabilities obtained by sum of state-to-state reaction probabilities (black dashed lines) and the ones calculated with a flux operator (colored solid lines) agree pretty well with each other, which indicates the convergence of the state-to-state reaction probabilities. The final product vibrational state-revolved state-to-state reaction probabilities can be used to decompose the total reaction probabilities into contributions from different vibrational states of the HD and OH products, as shown in Fig. 2 for the three modes of the DOH reactant. In panel (a) for reaction from DOH(000), both the (00) and (10) product vibrational states are present. Here, the two numbers in the bracket (ν1, ν2) denote the vibrational quantum numbers of the HD and OH products, respectively. Near the threshold energy of 0.85 eV, the HD and OH products are both in their vibrational ground states. When the collision energy increases by 0.45 eV above the threshold, the (10) product channel starts to appear due to the available energy to access the vibrationally excited HD state. This vibrationally excited HD product can be rationalized by the large coupling of the HD vibration to the reaction coordinate at the transition-state.58 Similarly for DOH(νOD = 1) in panel (b), the same two branches are present but with a lower threshold energy. However, no noticeable (01) state population is observed from the two initial states throughout the energy range shown in the figure despite the fact that it is energetically accessible.The decomposition of the total reaction probability from DOH(νOH = 1) is more interesting, as shown in Fig. 2(c). As expected for an OH spectator, both the (01) and (11) product vibrational states are populated starting from the same threshold as from DOH(000). Surprisingly, also present are the (00) and (10) product states, both with the OH product in its ground vibrational state, thus contradicting the spectator assumption for this mode. This accounts for the contribution to the total reaction probability in Fig. 1 at lower collision energies with a threshold of 0.4 eV. Furthermore, the feature in the total reaction probability from DOH(νOH = 1) is clearly why two segments with different slopes are present because they are contributed by different vibrational states of the OH product.
C. Vibrational mode specificity in differential cross sections
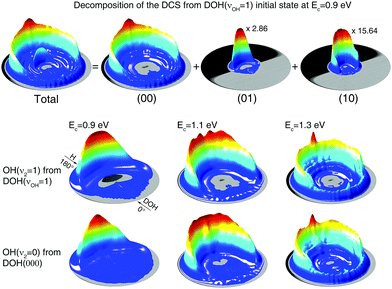
The differential cross section (DCS) represents the most detailed measurement of reaction dynamics, which resolves the angular distribution of the product ro-vibrational states, as shown in Fig. 3 as three-dimensional polar plots. The scattering angle is defined as the outgoing direction of the HD product relative to the direction of incoming H reactant. The radius in the plot represents the translational energy of the HD product in the center of mass frame, correlating to different ro-vibrational states of the HD and OH products by the laws of energy and momentum conservation.Similar to the decomposition of the total reaction probability, the DCS can also be decomposed into contributions from different vibrational states of the HD and OH products, as shown in the upper panel of Fig. 3 for the DCS from DOH(νOH = 1) at the collision energy of 0.9 eV. At this collision energy, only the (00), (10), and (01) product states are energetically accessible, as shown in Fig. 2(c), so that the DCS at this energy is decomposed into the contributions from these three product states. It is clearly seen that the DCS is dominated by the (00) vibrational branch while the (01) state population is ∼3 times smaller, and that from the (10) state is ∼15 times smaller and is obscured by the contribution from the (01) state close in energy. Among them, the (01) product is expected from the spectator model. Since we are mostly interested in the spectator character of the non-reacting OH bond, we will focus on the (01) contribution. In the lower panel of Fig. 3, individual contributions of the (01) product channel from DOH(νOH = 1) and the (00) product channel from DOH(000) are compared at three collision energies. Generally, DCSs at the same collision energy are similar. At Ec = 0.9 eV, they are both peaked at the backward direction, which is consistent with the direct abstraction reaction mechanism. With the increase of collision energy, broader angular distributions start to appear but they are all backward centered. At Ec = 1.3 eV, noticeable forward distributions are both clearly seen from the two initial states. These results suggest that the non-reacting OH bond is well behaved as a spectator above 0.9 eV, as it not only sequesters the original vibrational energy but also shows nearly identical angular distributions in both cases.52
The interesting discovery in Fig. 2(c) indicates that the OH(ν2 = 0) product is produced from DOH(νOH = 1). This apparently contradicts the well-accepted OH spectator picture because the vibrational excitation of the OH mode in the DOH reactant is not carried over to the OH product. This surprising observation is worth some further investigation.
As can be seen in Fig. 2, at the energy within 0.4 eV above the threshold, only the (00) product vibrational state is produced for all the three initial states. This energy range is between 0.7 and 1.1 eV in total energy. To gain further insights, we compare in Fig. 4 DCSs from the three initial states at three total energies in this range. Surprisingly, at the same total energy, the DCSs show similar angular distributions even though the initial states have quite different vibrational features. The resemblance of DCSs between the DOH(000) and DOH(νOD = 1) states is understandable, as the reaction proceeds by channeling either translational or vibrational energy into the reaction coordinate. Since the OD stretching mode is an active mode with strong coupling with the reaction coordinate, the energy initially deposited into the OD stretching mode is efficiently used to promote the reaction by channeling its energy into the reaction coordinate and thus the memory of initial excitation is lost during the reaction.55 This is the well-known ‘loss of memory’ effect of an active mode.59 With the same threshold but different reaction probabilities between the DOH(000) and DOH(νOD = 1) states, as shown in Fig. 1(b), the reaction can be understood in such a picture that the same amount of translational and the OD vibrational energies can both facilitate the reaction but they differ by their efficacy in promoting the reaction, i.e., different amounts of incoming reactant flux that can pass the barrier. For the reactive flux that passed the barrier, the final state distribution is determined largely by the coupling between the product modes and the reaction coordinate at the transition state.52,57,60,61 Due to the loss of initial state memory, it is not surprising to see similar angular distributions of product states in the DCSs from the two initial states.
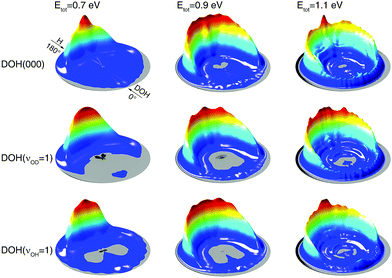 | ||
| Fig. 4 Three-dimensional polar plot of the DCSs from the DOH(000), DOH(νOD = 1), and DOH(νOH = 1) initial vibrational states at three total energies of 0.7, 0.9, and 1.1 eV. | ||
On the other hand, the close resemblance of DCSs between the DOH(νOD = 1) and DOH(νOH = 1) initial states in Fig. 4 cannot be explained by the ‘loss of memory’ effect mentioned above, as the non-reacting OH bond is not an active mode. As shown in Fig. 2 the non-reacting OH bond does not behave as a spectator mode at the three total energies shown. To this end, it is interesting to see the angular distributions of the DCSs from DOH(νOH = 1) and DOH(νOD = 1) resemble each other, although the initial vibrational excitations are distinctly different.
Discussion
The above results display two seemingly conflicting behaviors of the non-reacting OH bond in reaction from the DOH(νOH = 1) initial state. At high energies, the non-reacting OH bond is shown to behave largely as a spectator by preserving the original vibrational excitation and showing almost same angular distribution in the DCS of OH(ν2 = 1) from the DOH(νOH = 1) as the OH(ν2 = 0) one from DOH(000). However, there also exists an unexpected channel evidently at low energies that the OH vibrational excitation is ‘quenched’ to form OH(ν2 = 0) product from DOH(νOH = 1).One straightforward and possible explanation of the surprising result of the OH(ν2 = 0) product from DOH(νOH = 1) reactant is vibrational non-adiabaticity.62–66 To this end, the coupling of the non-reacting mode with active ones allows energy flow between them in the entrance channel, resulting in energy loss in the former. For instance, the 100% threshold reduction in the theoretical investigation of the H + HCN(νCN = 1) → H2 + CN reaction was attributed to vibrational non-adiabaticity.63 In Fig. 1, all the vibrational excitation energy of DOH(νOH = 1) is used to reduce the threshold. In addition, the similar vibrational excitation of the CN product from HCN(004) and HCN(302) reacting with Cl atom was explained by vibrational non-adiabaticity as well.67 Here in Fig. 4, the angular distribution of the DCS of DOH(νOH = 1) resembles the one from DOH(νOH = 1). These two observations both suggest the vibrational non-adiabaticity as the mechanism of the surprising result. According to this mechanism, the energy initially deposited in the OH stretching mode of the DOH reactant leaks out as H approaches, and the energy is used to overcome the reaction barrier. This results in the loss of vibrational excitation in the OH stretching mode, and subsequently the OH product in the ground vibrational state. This mechanism also predicts a lower reaction threshold. The OH stretching mode has a higher internal energy than the OD stretching mode by about 0.1 eV. After transferring the OH stretching energy to the OD stretching mode, there is an extra of 0.1 eV energy behaving as the translational energy. However, there is no direct evidence indicating energy leakage from DOH(νOH = 1).
To further investigate this issue, it is helpful to work in the local-mode regime,68 which provides a natural paradigm for describing the vibrations in DOH and studying vibrational non-adiabaticity. Specifically, the three DOH vibrational states studied here are projected on to six local-mode stretching bases with the assumption that the bending is decoupled, and their weights are listed in Table 1. The two numbers in the local-mode states |(nm)〉 denote the stretching vibrational quantum numbers of the OD and OH bonds, respectively. It is shown in Table 1 that the (000) eigenstate is almost purely represented by the |(00)〉 local-mode state. Surprisingly, however, the DOH(νOD = 1) and DOH(νOH = 1) eigenstates are not solely represented by the |(10)〉 and |(01)〉, i.e., OD and OH stretching local-mode states, respectively. For example, the DOH(νOD = 1) eigenstate has a 11.37% contribution from the |(00)〉 local-mode state, while the DOH(νOH = 1) eigenstate has a contribution of 0.96% from the |(10)〉 local-mode state. It should be noted here that albeit small the |(10)〉 local-mode component might contribute remarkably due to its large reactivity.

| (νOD, νb, νOH) | |Cnm|2 | |||||
|---|---|---|---|---|---|---|
| |(00)〉 | |(10)〉 | |(01)〉 | |(20)〉 | |(02)〉 | |(11)〉 | |
| (000) | 99.66 | 0.18 | 0.15 | 0.00 | 0.00 | 0.00 |
| (100) | 11.37 | 87.54 | 0.58 | 0.36 | 0.00 | 0.15 |
| (001) | 0.22 | 0.96 | 98.32 | 0.02 | 0.32 | 0.15 |
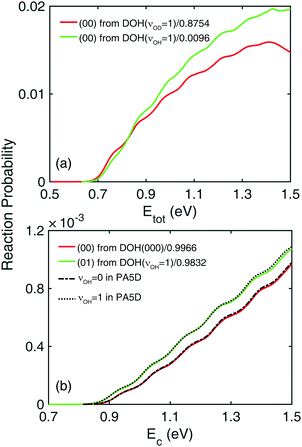
This interesting observation suggests that the small OD stretching (|(10)〉) local-mode component of the DOH(νOH = 1) state might actually contribute to the reactivity. In other words, the OD excited local-mode component (|(10)〉) in the DOH(νOH = 1) eigenstate will proceed to generate HD + OH with OH necessarily in the ground vibrational state. To demonstrate this possibility, the (00) product vibrational state-resolved reaction probabilities from the DOH(νOD = 1) and DOH(νOH = 1) initial states are plotted in Fig. 5(a), with normalization factors chosen as the weights of the |(10)〉 local-mode component in these two initial wavefunctions. These two curves are indeed quite similar, indicating that the contributing OD stretching is originated from the component already in the initial wave function, rather than from energy transferred from the OH stretching through vibrational non-adiabaticity. The contribution of the |(00)〉 and |(01)〉 local-mode components are negligible due to their small reactivity. At higher total energy above 1.0 eV, the difference between the two can be attributed to the fact that the |(20)〉 local-mode component also contributes to the forming of the (00) product states. This analysis argues against the vibrational non-adiabatic model, and attributes the additional reactivity to the local-mode excitation in the initial wavefunction.
Another piece of evidence against vibrational non-adiabaticity is from the missing of the OH(ν2 = 1) product from the DOH(νOD = 1) initial state, as shown in Fig. 2(b). If the vibrational non-adiabaticity were operative, energy initially deposited in the OD stretching mode could also flow into the OH stretching mode through non-adiabatic coupling. After passing the transition state, the excited OH stretching mode would result in the OH(ν2 = 1) product.
To further provide evidence against vibrational non-adiabaticity, we take advantage of a reduced-dimensional model in which the non-reacting OH bond is excluded from the dynamical model, but its effect is considered as mean-field contribution through potential averaging by the one-dimensional νOH = 0 or 1 stretching vibrational wavefunction. In this way, the OH stretching mode in DOH is purely a local mode and fixed at one of the vibrational states throughout the reaction. This PA5D method has been shown to be quite accurate for this reaction when the reactant is in its ground vibrational state.26 If vibrational non-adiabaticity were present, the PA5D results should be different from those obtained in full-dimensionality, because the former disallows energy flow into or out of the OH mode. To make a meaningful comparison, the reaction probabilities from the DOH(νOD = 1) and DOH(νOH = 1) initial states are again normalized with the weights of the appropriate local-mode components. In addition, a small energy shift of 0.003 eV is added, because the eigenstate of DOH reactant in the PA5D approximation is about 0.003 eV higher than the full-dimensional one. As shown in Fig. 5(b), the agreement between full- and reduced-dimensional results is excellent for both νOH = 0 and 1, ruling out vibrational non-adiabaticity as a possible mechanism for the H + DOH(νOH = 1) → HD + OH(ν2 = 0) reaction.
It should be noted that the PA5D total reaction probability from the DOH(νOH = 1) initial state is quite different from the one shown in Fig. 1. The discrepancy is not due to the failure of this approximation but to the inaccuracy in representing the initial vibrational state of the DOH reactant, i.e., the missing of |(10)〉 and (|(00)〉) local-mode components. On the other hand, the total reaction probabilities from the DOH(000) initial state are similar between the full-dimensional calculation and the PA5D approximation. This is because the DOH(000) eigenstate is well represented in both cases due to the negligible vibrational excitation in the OH bond. Up to now, the PA5D approximation has only applied to the case when the spectator OH is in the ground vibrational state.26 There is no limitation in applying this model to vibrationally excited OH spectator, but extra care should be taken to account for the initial eigenstate in full dimensionality.
In summary, the analysis above rules out vibrational non-adiabaticity as the origin of the OH(ν2 = 0) production from the H + DOH(νOH = 1) → HD + OH(ν2 = 0) reaction. Instead, it originates from a small component of the OD excited local-mode in the DOH(νOH = 1) initial wavefunction. The small weight of this local-mode component is compensated by its large reactivity.
Conclusions
In this work, we report a detailed analysis of the unexpected discovery of the OH(ν2 = 0) product from the DOH(νOH = 1) initial state in the title reaction, using a state-to-state full-dimensional quantum dynamical method on an accurate PES. By taking advantage of the local-mode analysis and the decomposition of both the reaction probability and differential cross sections in terms of the product vibrational states, we identify correlations between initial and final states of the reaction. Two possible mechanisms are explored. One envisions non-adiabatic energy leakage from the spectator OH mode during the reaction, while the other assumes some OD excitation in the initial state. Our extensive analysis leads us to rule out the former mechanism of vibrational non-adiabaticity as the main cause of the surprising reactivity. Instead, we demonstrate that the phenomenon is due to small OD stretching (|(10)〉) local-mode component in the DOH(νOH = 1) initial state. The large reactivity of the OD excited initial state compensates its small weight, rendering a substantial contribution. In other words, the mixing of states exists in the initial state rather than during the reaction.These results reaffirm that the non-reacting OH bond in the DOH reactant behaves perfectly as a spectator no matter it is initially prepared in the vibrational ground or first excited state. This lends support to the commonly accepted notion that for reactions involving larger molecules, the site of reaction only contains a small number of active modes and the remaining ones are all behaved largely as spectators.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Z. B. thanks Alexander von Humboldt-Stiftung for a Fellowship and Prof. Uwe Manthe for useful discussions. This work was supported by the US Department of Energy (Grant No. DE-SC0015997 to H. G.) and by the National Natural Science Foundation of China (Grant No. 21222308, 21133006 and 21433009 to Z. S.). Part of the calculations were performed at the Center for Advance Research Computing (CARC) at University of New Mexico.References
- S. C. Althorpe and D. C. Clary, Annu. Rev. Phys. Chem., 2003, 54, 493–529 CrossRef CAS PubMed.
- W. Hu and G. C. Schatz, J. Chem. Phys., 2006, 125, 132301 CrossRef PubMed.
- D. C. Clary, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12649 CrossRef CAS PubMed.
- D. H. Zhang and H. Guo, Annu. Rev. Phys. Chem., 2016, 67, 135–158 CrossRef CAS PubMed.
- K. Liu, Adv. Chem. Phys., 2012, 149, 1–46 CAS.
- B. Joalland, Y. Shi, A. D. Estillore, A. Kamasah, A. M. Mebel and A. G. Suits, J. Phys. Chem. A, 2014, 118, 9281–9295 CrossRef CAS PubMed.
- K. Liu, Annu. Rev. Phys. Chem., 2016, 67, 91–111 CrossRef CAS PubMed.
- F. F. Crim, Acc. Chem. Res., 1999, 32, 877–884 CrossRef CAS.
- M. A. Collins, Theor. Chem. Acc., 2002, 108, 313–324 CrossRef CAS.
- J. M. Bowman, G. Czakó and B. Fu, Phys. Chem. Chem. Phys., 2011, 13, 8094–8111 RSC.
- B. Jiang, J. Li and H. Guo, Int. Rev. Phys. Chem., 2016, 35, 479–506 CrossRef CAS.
- B. Zhao and H. Guo, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2017, 7, e1301 CrossRef.
- J. Li, B. Jiang, H. Song, J. Ma, B. Zhao, R. Dawes and H. Guo, J. Phys. Chem. A, 2015, 119, 4667–4687 CrossRef CAS PubMed.
- A. Sinha, M. C. Hsiao and F. F. Crim, J. Chem. Phys., 1991, 94, 4928–4935 CrossRef CAS.
- A. Sinha, J. D. Thoemke and F. F. Crim, J. Chem. Phys., 1992, 96, 372–376 CrossRef CAS.
- M. J. Bronikowski, W. R. Simpson and R. N. Zare, J. Phys. Chem., 1993, 97, 2194–2203 CrossRef CAS.
- M. J. Bronikowski, W. R. Simpson and R. N. Zare, J. Phys. Chem., 1993, 97, 2204–2208 CrossRef CAS.
- B. R. Strazisar, C. Lin and H. F. Davis, Science, 2000, 290, 958–961 CrossRef CAS PubMed.
- I. W. M. Smith and F. F. Crim, Phys. Chem. Chem. Phys., 2002, 4, 3543 RSC.
- C. Xiao, X. Xu, S. Liu, T. Wang, W. Dong, T. Yang, Z. Sun, D. Dai, X. Xu, D. H. Zhang and X. Yang, Science, 2011, 333, 440–442 CrossRef CAS PubMed.
- S. Liu, C. Xiao, T. Wang, J. Chen, T. Yang, X. Xu, D. H. Zhang and X. Yang, Faraday Discuss., 2012, 157, 101–111 RSC.
- R. P. A. Bettens, M. A. Collins, M. J. T. Jordan and D. H. Zhang, J. Chem. Phys., 2000, 112, 10162 CrossRef CAS.
- G.-S. Wu, G. C. Schatz, G. Lendvay, D. C. Fang and L. B. Harding, J. Chem. Phys., 2000, 113, 3150–3161 CrossRef CAS.
- M. Yang, D. H. Zhang, M. A. Collins and S.-Y. Lee, J. Chem. Phys., 2001, 115, 174–178 CrossRef CAS.
- J. Chen, X. Xu, X. Xu and D. H. Zhang, J. Chem. Phys., 2013, 138, 154301 CrossRef PubMed.
- D. H. Zhang and J. Z. H. Zhang, J. Chem. Phys., 1994, 101, 1146–1156 CrossRef CAS.
- D. Neuhauser, J. Chem. Phys., 1994, 100, 9272–9275 CrossRef CAS.
- U. Manthe, T. Seideman and W. H. Miller, J. Chem. Phys., 1993, 99, 10078–10081 CrossRef CAS.
- J. Dai, W. Zhu and J. Z. H. Zhang, J. Phys. Chem., 1996, 100, 13901–13903 CrossRef CAS.
- W. Zhu, J. Dai, J. Z. H. Zhang and D. H. Zhang, J. Chem. Phys., 1996, 105, 4881–4884 CrossRef CAS.
- D. H. Zhang and J. C. Light, J. Chem. Phys., 1996, 104, 4544–4553 CrossRef CAS.
- D. H. Zhang and J. C. Light, J. Chem. Phys., 1996, 105, 1291–1294 CrossRef CAS.
- D. H. Zhang and J. C. Light, J. Chem. Soc., Faraday Trans., 1997, 93, 691–697 RSC.
- D. H. Zhang, M. A. Collins and S.-Y. Lee, Science, 2000, 290, 961–963 CrossRef CAS PubMed.
- D. H. Zhang, M. Yang and S.-Y. Lee, J. Chem. Phys., 2001, 114, 8733 CrossRef CAS.
- D. H. Zhang, D. Xie and M. Yang, Phys. Rev. Lett., 2002, 89, 283203 CrossRef PubMed.
- E. M. Goldfield and S. K. Gray, J. Chem. Phys., 2002, 117, 1604 CrossRef CAS.
- P. Defazio and S. K. Gray, J. Phys. Chem. A, 2003, 107, 7132–7137 CrossRef CAS.
- J. Mayneris, M. Gonzalez and S. K. Gray, Comput. Phys. Commun., 2008, 179, 741–747 CrossRef CAS.
- M. T. Cvitaš and S. C. Althorpe, J. Phys. Chem. A, 2009, 113, 4557 CrossRef PubMed.
- M. T. Cvitaš and S. C. Althorpe, J. Chem. Phys., 2011, 134, 024309 CrossRef PubMed.
- B. Jiang, D. Xie and H. Guo, J. Chem. Phys., 2011, 135, 084112 CrossRef PubMed.
- M. T. Cvitaš and S. C. Althorpe, J. Chem. Phys., 2013, 139, 064307 CrossRef PubMed.
- B. Fu and D. H. Zhang, J. Chem. Phys., 2013, 138, 184308 CrossRef PubMed.
- B. Zhao, Z. Sun and H. Guo, J. Chem. Phys., 2014, 141, 154112 CrossRef PubMed.
- B. Fu and D. H. Zhang, J. Chem. Phys., 2015, 142, 064314 CrossRef PubMed.
- B. Zhao, Z. Sun and H. Guo, J. Chem. Phys., 2016, 144, 064104 CrossRef PubMed.
- B. Zhao, Z. Sun and H. Guo, J. Chem. Phys., 2016, 144, 214303 CrossRef PubMed.
- S. Liu, X. Xu and D. H. Zhang, J. Chem. Phys., 2012, 136, 144302 CrossRef PubMed.
- S. Liu and D. H. Zhang, Chem. Sci., 2016, 7, 261–265 RSC.
- Z. Zhao, S. Liu and D. H. Zhang, J. Chem. Phys., 2016, 145, 134301 CrossRef PubMed.
- B. Zhao, Z. Sun and H. Guo, J. Chem. Phys., 2016, 145, 134308 CrossRef PubMed.
- B. Zhao, Z. Sun and H. Guo, J. Chem. Phys., 2016, 145, 184106 CrossRef PubMed.
- J. C. Polanyi, Science, 1987, 236, 680–690 CAS.
- H. Guo and K. Liu, Chem. Sci., 2016, 7, 3992–4003 RSC.
- H. Guo and B. Jiang, Acc. Chem. Res., 2014, 47, 3679–3685 CrossRef CAS PubMed.
- B. Zhao, Z. Sun and H. Guo, J. Am. Chem. Soc., 2015, 137, 15964–15970 CrossRef CAS PubMed.
- B. Jiang and H. Guo, J. Am. Chem. Soc., 2013, 135, 15251–15256 CrossRef CAS PubMed.
- R. Welsch and U. Manthe, J. Phys. Chem. Lett., 2015, 6, 338–342 CrossRef CAS PubMed.
- B. Zhao, Z. Sun and H. Guo, J. Chem. Phys., 2015, 142, 241101 CrossRef PubMed.
- C. Xie, B. Jiang, M. Yang and H. Guo, J. Phys. Chem. A, 2016, 120, 6521–6528 CrossRef CAS PubMed.
- W. T. Duncan and T. N. Truong, J. Chem. Phys., 1995, 103, 9642–9652 CrossRef CAS.
- T. Takayanagi and G. C. Schatz, J. Chem. Phys., 1997, 106, 3227–3236 CrossRef CAS.
- L. Halonen, S. L. Bernasek and D. J. Nesbitt, J. Chem. Phys., 2001, 115, 5611–5620 CrossRef CAS.
- S. Yoon, S. Henton, A. N. Zivkovic and F. F. Crim, J. Chem. Phys., 2002, 116, 10744–10752 CrossRef CAS.
- S. Yan, Y.-T. Wu and K. Liu, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12667–12672 CrossRef CAS PubMed.
- J. M. Pfeiffer, R. B. Metz, J. D. Thoemke, E. Woods and F. F. Crim, J. Chem. Phys., 1996, 104, 4490–4501 CrossRef CAS.
- M. S. Child and L. Halonen, Adv. Chem. Phys., 1984, 57, 1–58 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Parameters used in the calculations and additional results. See DOI: 10.1039/c7cp07199j |
| ‡ Present address: Theoretische Chemie, Fakultät für Chemie, Universität Bielefeld, Universitätsstr. 25, D-33615 Bielefeld, Germany. |
| This journal is © the Owner Societies 2018 |