Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High CO2 absorption capacity by chemisorption at cations and anions in choline-based ionic liquids†
Shubhankar
Bhattacharyya
 *a,
Andrei
Filippov
*a,
Andrei
Filippov
 *ab and
Faiz Ullah
Shah
*ab and
Faiz Ullah
Shah
 a
a
aChemistry of Interfaces, Luleå University of Technology, Luleå SE-97187, Sweden. E-mail: shubhankar.bhattacharyya@ltu.se; andrei.filipov@ltu.se
bInstitute of Physics, Kazan Federal University, 420008 Kazan, Russia
First published on 2nd November 2017
Abstract
The effect of CO2 absorption on the aromaticity and hydrogen bonding in ionic liquids is investigated. Five different ionic liquids with choline based cations and aprotic N-heterocyclic anions were synthesized. Purity and structures of the synthesized ionic liquids were characterized by 1H and 13C NMR spectroscopy. CO2 capture performance was studied at 20 °C and 40 °C under three different pressures (1, 3, 6 bar). The IL [N1,1,6,2OH][4-Triz] showed the highest CO2 capture capacity (28.6 wt%, 1.57 mol of CO2 per mol of the IL, 6.48 mol of CO2 per kg of the ionic liquid) at 20 °C and 1 bar. The high CO2 capture capacity of the [N1,1,6,2OH][4-Triz] IL is due to the formation of carbonic acid (–OCO2H) together with carbamate by participation of the –OH group of the [N1,1,6,2OH]+ cation in the CO2 capture process. The structure of the adduct formed by CO2 reaction with the IL [N1,1,6,2OH][4-Triz] was probed by using IR, 13C NMR and 1H–13C HMBC NMR experiments utilizing 13C labeled CO2 gas. 1H and 13C PFG NMR studies were performed before and after CO2 absorption to explore the effect of cation–anion structures on the microscopic ion dynamics in ILs. The ionic mobility was significantly increased after CO2 reaction due to lowering of aromaticity in the case of ILs with aromatic N-heterocyclic anions.
Introduction
Ionic liquids (ILs) are typically low melting salts (usually <100 °C) comprising organic or inorganic cations and anions. Unique physicochemical properties such as high ionic conductivity, low vapor pressure, non-flammability and functional designability make ILs suitable sorbents for CO2 as compared to the conventional molecular liquids. Though ILs have a wide range of properties, however, viscosity is always a major concern when they are used as CO2 sorbents. Due to the presence of cations and anions in the same system, ILs are usually more viscous than other conventional molecular liquids.1–3 Often ILs are regarded as designer solvents due to their tailor-made physicochemical properties by the manipulation of cation and anion structures. Extensive research efforts have already been made to understand and predict the macroscopic physicochemical properties of the ILs on the basis of their ionic interactions, microscopic ion dynamics, etc. Despite the extensive research efforts, the influence of cationic and anionic structures on the physicochemical properties of the ILs still remains a challenge. Thus, investigation of the ionic mobility of ILs is a promising area of research for the development of high performance ILs for challenging applications.4,5Diffusivity (also known as self-diffusion) of molecules, ions and their aggregates is a property of any liquid as a result of thermal motion.6 This property determines many of the physicochemical characteristics of a liquid such as viscosity, ionic conductivity, and adsorption, and also influences the mechanisms and rates of chemical reactions. In this regard, investigation of the diffusivity of ILs can help in gaining new insights and a better understanding of their ion dynamics, transport properties7,8 and other technological processes.7 Self-diffusion in ILs generally obeys the laws of diffusivity of molecules in liquids such as Gaussian statistics for mean-squared displacements and the Stokes–Einstein equation.6 Moreover, self-association phenomena are quite typical for many ILs9 that lead to a specific diffusivity of ions.10–12,37 Absorption of gases is another type of association that also effects the diffusivity of ions.13 Nuclear magnetic resonance (NMR) spectroscopy is an appropriate technique for the investigation of the diffusion of ions and sorbent additives in the ILs.14
Because of the prevalent industrial applications of ILs, they are also regarded as promising CO2 sorbents. However, the higher viscosity of ILs compared to the conventional amine sorbents remains a challenge, limiting their applicability as CO2 sorbents. Recently, great attention has been paid by researchers to develop less viscous IL CO2 sorbents as a possible replacement for the conventional amine sorbents. The most promising ILs as CO2 sorbents are amino acid based ionic liquids with phosphonium and ammonium cations.15 One of the possible reasons is that amino acid based ILs have low toxicity and faster reactivity towards CO2 molecules. Recently we have reported that functionalized choline based amino acid ILs have excellent CO2 capture capacity at room temperature and atmospheric pressure.16 Additionally, the ILs with choline based cations are more attractive due to their low toxicity and biodegradability.17–19 At the same time, ILs containing amino acid anions with simple choline cations are highly viscous due to hydrogen bonding. We have found that etherification of choline cations leads to a dramatically low viscosity.16 Also by replacing amino acid anions with N-heterocyclic anions, the viscosity of the ILs decreased significantly with improved CO2 capture capacity.13 In addition, it was observed that most of the amino acid based ILs become highly viscous or solidified after CO2 absorption. However, aprotic N-heterocyclic anion (AHA) based ILs remained liquid with much faster ionic mobility after reaction with CO2 molecules. Detailed insights into the comparative physicochemical and CO2 capture studies of amino acid and N-heterocyclic amine based ILs with a common cation [N1,1,6,2OH]+ are recently reported.13
CO2 capture studies of some aprotic N-heterocyclic based anions with a phosphonium cation [P6,6,6,14]+ have already been reported by different research groups. For example, AHA based IL phosphonium cations were reported by Wang et al. who achieved a CO2 capture capacity of up to 7.5 wt% (0.95 mol CO2/IL) using [P6,6,6,14][4-Triz].38 Then using the same phosphonium cation [P6,6,6,14]+, Brennecke's group reported on phosphonium indazolate [P6,6,6,14][Inda] based ILs with a maximum CO2 absorption capacity of 7.1 wt% (0.98 mol CO2/IL) under ambient pressure and temperature.39 The regeneration of ILs often needs heating (up to 80 °C) with a continuous flow of nitrogen gas into the system. Furthermore, these CO2 absorption capacities are significantly lower as compared to that of the traditional 2-aminoethanol (MEA or monoethanolamine) that has 29.9 wt% (0.42 mol) as a neat sorbent and 13.1 wt% capacity can also achieved by using 30% aqueous 2-aminoethanol (MEA) solution.42 There is an urgent need to replace 2-aminoethanol with environmentally benign ILs having higher CO2 capture capacity.
Apart from AHA based ILs, various protic ILs have also been reported for the CO2 capture process. The major advantage of protic ILs is their low viscosity compared to AHA based ILs. However, CO2 capture capacity and the process of regeneration of ILs have still not been improved. Zhu et al. recently reported on new 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) based protic ILs for CO2 capture. However, the maximum CO2 absorption capacity of DBU based protic ILs obtained for the IL [DBUH+][Im−] was ∼18 wt% (0.9 mol CO2/IL).40 Similarly, Dai et al. also reported 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (MTBD) superbase-derived protic IL [MTBDH+][Im−] and showed a CO2 absorption capacity of 17.0 wt% (1.03 mol CO2 per mol of IL).41 CO2 capture by diamino based protic ILs has been reported by MacFarlane's group, although the maximum CO2 uptake achieved was 12.6 wt% (0.47 mol CO2 per mol of IL).42 The CO2 capture capacity of these ILs is not substantial (<20 wt%) compared to that of the traditional 2-aminoethanol (MEA) which also requires the energy demanding regeneration process to recycle the ILs. Therefore, there is a continuous quest for the development of new ionic liquids with high and reversible CO2 absorption capacity with minimum energy input.
In this work, we investigate a comparison of the physiochemical properties as well as CO2 capture capacities of different N-heterocyclic anion based ILs with functionalized choline as the cation. Five ILs having choline based cations and different aprotic N-heterocyclic anions were synthesized and characterized. The functionalized choline cation [N1,1,6,2OH] is further modified by etherification to study the effect of hydrogen bonding on the CO2 absorption capacity, viscosity and ion mobility. FTIR, 1H and 13C NMR spectroscopic techniques were employed to characterize the structure of the adduct formed as a result of CO2 reaction with the IL. In addition, 1H NMR self-diffusion measurements were carried out on neat ionic liquids as well as after CO2 absorption experiments using 1H and 13C pulsed field gradient (PFG) NMR techniques to understand the ionic mobility in these ILs. Density functional theory (DFT) calculations were performed to further support the experimental findings of the effect of CO2 absorption on the ionic mobility of the ionic liquids.
Synthesis and characterization
All the reactants were purchased from Sigma Aldrich and used without further purification. Thin layer chromatographic analysis was carried out using silica gel 60 Å F-254 TLC plates with KMnO4 charring solution.All the ionic liquids were prepared using our previously reported procedures.13 The excess of unreacted N-heterocycles was removed by adding excess of ACN and was stored over night at 0 °C. All the ILs were dried under vacuum at 60 °C for at least 2 days.
The 1H and 13C NMR characterization data for the synthesized ILs are given in the ESI.†
Water content was measured using a Metrohm 917 Karl Fischer Coloumeter with a HYDRANAL reagent. It was found that all ILs contain a water content of <0.5%.
Physicochemical characterization
The thermogravimetric analysis (TGA) of all the five ILs was performed using a Perkin Elmer 8000 TGA instrument in the temperature range from 30 °C to 550 °C with a heating ramp of 10 °C min−1 under a nitrogen atmosphere. 2–3 mg of the ionic liquid sample was used for each experiment. All the TGA experiments were performed under nitrogen gas as the inert carrier gas.A Lovis 2000 ME Automated Microviscometer (Anton-Paar falling ball type viscometer with 2.50 mm glass capillary, viscosity range 10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cP) was used for viscosity measurement. The viscosity of the ILs was measured in the temperature range from 20 °C to 80 °C with a step size of 5 °C using a sealed glass capillary sample tube.
000 cP) was used for viscosity measurement. The viscosity of the ILs was measured in the temperature range from 20 °C to 80 °C with a step size of 5 °C using a sealed glass capillary sample tube.
NMR and FTIR spectroscopy
A Bruker Ascend Aeon WB 400 (Bruker BioSpin AG, Fällanden, Switzerland) NMR spectrometer was used with a working frequency of 400.22 MHz for 1H and 100.65 MHz for 13C. Bruker Topspin 3.5 software was used for the processing of data. All the spectra were recorded at 25 °C. Chemical shifts were expressed in parts per millions (δ) downfield from DSS with the solvent resonance as the internal standard (D2O, δ = 4.79) and were reported as s (singlet), d (doublet), t (triplet), q (quartet), br (broad) and m (multiplet). All coupling constants (J) are reported in Hertz (Hz).The 1H and 13C pulsed field gradient (PFG) NMR self-diffusion experiments were performed on a Bruker Ascend Aeon WB 400 instrument using a Diff50 (60 A) z-gradient 5 mm diffusion probe. A stimulated echo pulse sequence was employed,14,20 with the diffusion coefficient (D) determined from the signal decay using the Stejskal and Tanner equation20
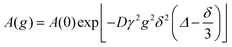 | (1) |
Fourier transform infrared (FTIR) spectra were recorded on a Bruker IFS 80v vacuum Fourier transform infrared spectrometer equipped with a deuterated triglycine sulphate (DTGS) detector. All spectra were recorded at room temperature (∼22 °C) using the double side forward–backward acquisition mode. A total number of 256 scans were co-added and signal-averaged at an optical resolution of 4 cm−1.
CO2 absorption measurement
CO2 capture experiments were performed using ∼0.1 g of neat ILs in a 10 ml Buchi stainless steel reactor with a PTFE insert and a pressure gauge. The CO2 capture capacities of the ILs were measured at different pressures (1–6 bar) of CO2 (99.999 by weight%) at 20 °C and 40 °C by weighing the PTFE insert gravimetrically using a Mettler Toledo analytical balance (0.1 mg accuracy).Computational methods
DFT calculations were performed using GAUSSIAN 0921 software packages. The B3LYP/6-311+g (d,p) level of theory was used for molecular geometry optimization. The optimized molecular geometries were characterized to be a relative energy minimum to the potential energy surface by real frequency. For NICS calculations the aug-cc-pvtz basis set was used. However, all calculations are performed under gas phase conditions, in the current calculations cations and the solvent model are not included as the solvent properties are not available for the IL structure.Results and discussion
Five ILs with four different aprotic heterocyclic anions (imidazole, triazole, pyrazole, succinimide) were synthesized by following our previously reported procedures.13 A choline based cation [N1,1,6,2OH]+ and a choline based ether functionalized cation [N1,1,6,2O4]+ are selected to investigate the effect of hydrogen bonding and ether functionalization on the ionic mobility before and after CO2 absorption. To further investigate the effect of non-aromatic heterocyclic anions on the physicochemical properties of ILs, the [N1,1,6,2OH][Succ] ionic liquid with a common cation [N1,1,6,2OH]+ and a succinimide anion was selected. Furthermore, the effect of ether functionalization on the cation was also studied using an ether functionalized ionic liquid [N1,1,6,2O4][4-Triz] in comparison with a non-ether functionalized ionic liquid [ N1,1,6,2OH][4-Triz] having a common 1,2,4-triazolate anion [4-Triz]−. The chemical structures and abbreviations of the ionic components of the studied ionic liquids are shown in Fig. 1.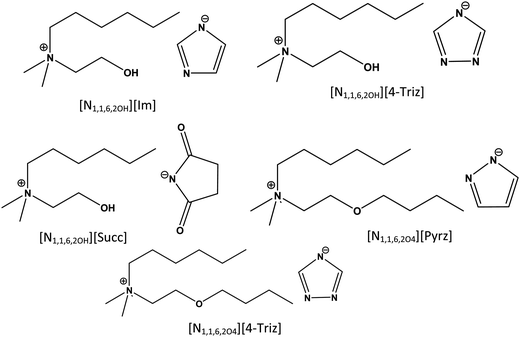 | ||
| Fig. 1 Chemical structures and abbreviations of the ionic components of the choline based N-heterocyclic ionic liquids investigated. | ||
Viscosity is one of the most influential physiochemical properties of ionic liquids used for various applications. Viscosity of the four different ionic liquids [N1,1,6,2OH][Im], [N1,1,6,2OH][4-Triz], [N1,1,6,2OH][Succ] and [N1,1,6,2O4][4-Triz], which were liquid at room temperature, was measured as a function of temperature (ESI,† S10). The ionic liquid [N1,1,6,2O4][Pyrz] was solid at room temperature. It was observed that viscosity of all these ILs is temperature dependent and can be fitted well into an Arrhenius form of equation for viscosity η = η0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[Ea(η)/kBT] for the measured temperature range from 20 °C to 80 °C, where η0 and Ea(η) are the constant and the activation energy for the viscous flow. From the Arrhenius equation for viscosity, the activation energy for the viscous flow was calculated. For less viscous ILs [N1,1,6,2OH][4-Triz] and [N1,1,6,2OH][Imi] the activation energies for viscous flow are 59.7 kJ mol−1 K−1 and 57.5 kJ mol−1 K−1, respectively. It is interesting to note that the activation energy for viscous flow significantly decreased to 50.6 kJ mol−1 K−1 upon ether functionalization of the cation [N1,1,6,2OH]. In addition, the activation energy for viscous flow increased significantly to 76.8 kJ mol−1 K−1 when an aromatic heterocyclic anion was replaced by a non-aromatic heterocyclic anion (succinimide anion).
exp[Ea(η)/kBT] for the measured temperature range from 20 °C to 80 °C, where η0 and Ea(η) are the constant and the activation energy for the viscous flow. From the Arrhenius equation for viscosity, the activation energy for the viscous flow was calculated. For less viscous ILs [N1,1,6,2OH][4-Triz] and [N1,1,6,2OH][Imi] the activation energies for viscous flow are 59.7 kJ mol−1 K−1 and 57.5 kJ mol−1 K−1, respectively. It is interesting to note that the activation energy for viscous flow significantly decreased to 50.6 kJ mol−1 K−1 upon ether functionalization of the cation [N1,1,6,2OH]. In addition, the activation energy for viscous flow increased significantly to 76.8 kJ mol−1 K−1 when an aromatic heterocyclic anion was replaced by a non-aromatic heterocyclic anion (succinimide anion).
It is observed that the IL with a non-aromatic heterocyclic anion (succinimide) was highly viscous at room temperature (≫10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cP) compared to the other ionic liquids with aromatic anions. The viscosity of [N1,1,6,2OH][Succ] was measurable only at temperature higher than 50 °C using a falling ball viscometer. Although at 50 °C, the viscosity of [N1,1,6,2OH][Succ] was very high >9000 cP. However, as expected the viscosity of [N1,1,6,2OH][Succ] decreased significantly with the increase in temperature. At higher temperatures 80 °C, the viscosity dropped significantly to 803.7 cP (ESI,† S10).
000 cP) compared to the other ionic liquids with aromatic anions. The viscosity of [N1,1,6,2OH][Succ] was measurable only at temperature higher than 50 °C using a falling ball viscometer. Although at 50 °C, the viscosity of [N1,1,6,2OH][Succ] was very high >9000 cP. However, as expected the viscosity of [N1,1,6,2OH][Succ] decreased significantly with the increase in temperature. At higher temperatures 80 °C, the viscosity dropped significantly to 803.7 cP (ESI,† S10).
Interestingly, the viscosity of ILs with aromatic heterocyclic anions was found to be significantly less as compared to ILs containing no N-heterocyclic anions with a common cation [N1,1,6,2OH]. For example, at 20 °C the viscosities of [N1,1,6,2OH][4-Triz] and [N1,1,6,2OH][Imi] were around 8953 cP and 4003 cP, respectively. With increasing temperature to 40 °C in the case of [N1,1,6,2OH][4-Triz], the viscosity decreased by 6 fold around 1570 cP, whereas for the IL [N1,1,6,2OH][Imi] the viscosity was less than 900 cP. Upon further increase in temperature to 60 °C, the viscosities of [N1,1,6,2OH][4-Triz] and [N1,1,6,2OH][Imi] decreased to 402.5 cP and 231 cP, respectively. At a higher temperature of 80 °C less difference in viscosity was observed for ILs [N1,1,6,2OH][4-Triz] and [N1,1,6,2OH][Imi]. The viscosity of the IL [N1,1,6,2OH][4-Triz] was always higher than that of the IL [N1,1,6,2OH][Imi] because the higher number of nitrogen atoms present in the triazole anion leads to stronger hydrogen bonding as compared to the imidazole anion.
To further investigate the effect of hydrogen bonding on the viscosity, we measured the viscosity of the ether functionalized IL [N1,1,6,2O4][4-Triz] in the same temperature range. From our previously reported work, we envision that etherification of choline based cations will lead to reduced H-bonding interactions resulting in less viscous ILs.16 As expected, the viscosity of [N1,1,6,2O4][4-Triz] was found to be much lower, 1673 cP, at 20 °C as compared with [N1,1,6,2OH][4-Triz]. Further, upon increasing the temperature to 50 °C the viscosity further decreased to 204 cP, which is lower than the previously reported viscosity for the IL with the same anion and the [P6,6,6,14]+ cation.22 It is probably due to a considerable hydrophobic interaction between the longer alkyl chains of the [P6,6,6,14][4-Triz] IL. At a high temperature of 80 °C the viscosity of [N1,1,6,2O4][4-Triz] was further lowered to 48 cP, whereas the viscosity of the IL [P6,6,6,14][4-Triz] was 69 cP. Although the viscosity of the IL decreased after ether functionalization, however, the IL [N1,1,6,2O4][4-Triz] became semi-solid after one day. In addition, we also studied the ether functionalized cation [N1,1,6,2O4] with a pyrazole anion. The IL [N1,1,6,2O4][Pyrz] was found to be a semi-solid at room temperature. This also indicates that hydrogen-bonding interactions play a key role in the solid–liquid behavior of ionic liquids. The viscosities of these choline-based ILs is still higher in comparison to those of protic ILs but most of the viscosity data are comparable with those of the previously reported AHA based ILs38,39 by different groups. In addition these current viscosity studies can enrich our knowledge to design choline based low viscous AHA ILs for future applications.
Thermogravimetric analysis implies that upon ether functionalization of choline based cations the thermal stability of the ILs is decreased. Thermal degradation of the ether functionalized IL [N1,1,6,2O4][4-Triz] occurred around ∼150 °C, whereas that of the non-ether functionalized IL [N1,1,6,2OH][4-Triz] occurred at ∼160 °C. This also suggests that hydrogen-bonding interactions play a significant role in the thermal stability of ILs. However, the IL [N1,1,6,2O4][Pyrz] showed a relatively lower thermal stability (∼120 °C). This is most probably due to the high reactivity of the nucleophilic pyrazole anion that decreases the thermal stability of this ionic liquid. The TGA curves are shown in the ESI† (S11).
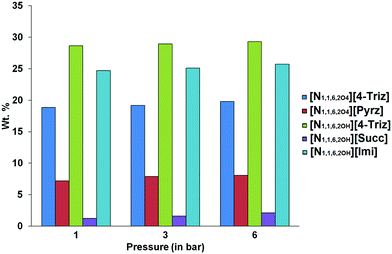
The CO2 capture performance of the five different ionic liquids was studied at 20 °C and 40 °C and three different pressures. The experiments were carried out gravimetrically with the neat ILs in closed pressure reactor with constant stirring. It is observed that the IL [N1,1,6,2OH][Succ] doesn’t react with CO2 even at elevated temperature (40 °C). This was confirmed by the absence of carbamate signal in the 13C NMR spectrum for the IL [N1,1,6,2OH][Succ] after the CO2 capture experiment. Most of the CO2 was physically absorbed and the CO2 solvation gradually increased with increasing pressure. At 20 °C under 6 bar pressure the CO2 capture capacity of the IL [N1,1,6,2OH][Succ] was 2.1 wt% (0.13 mol of CO2 per mol of the IL). Surprisingly, at elevated temperature (40 °C) the CO2 capture capacity of the IL [N1,1,6,2OH][Succ] increased by 3 fold (6.6 wt%, 0.4 mol of CO2 per mol of IL at 6 bar). It is probably due to the decrease in viscosity at 40 °C, which leads to faster diffusion of CO2 molecules in the IL (Fig. 2 and 3).
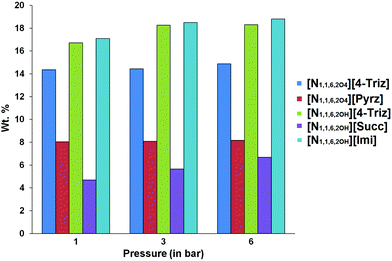
At 20 °C the IL [N1,1,6,2OH][4-Triz] showed the highest CO2 capture capacity at lower (28.6 wt%, 1.57 mol of CO2 per mol of IL at 1 bar) as well as higher pressure (29.3 wt%, 1.6 mol of CO2 per mol of IL at 6 bar). Although the IL [N1,1,6,2OH][Imi] showed a slightly lower CO2 capture capacity at 20 °C (24.7 wt%, 1.35 mol of CO2 per mol of IL at 1 bar), however, the CO2 capture capacity is increased to 25.7 wt% (1.4 mol of CO2 per mol of IL) at higher pressure (6 bar). In order to further investigate the effect of ether functionalization of the [N1,1,6,2OH]+ cation, we performed the CO2 capture experiment of the ether functionalized IL [N1,1,6,2O4][4-Triz]. Surprisingly, after ether functionalization the CO2 capture capacity decreased significantly. The CO2 uptake capacity of the IL [N1,1,6,2O4][4-Triz] was 18.8 wt% (1.2 mol CO2 per mol of IL) at 20 °C and 1 bar ambient pressure (Fig. 2). A slight increase in the CO2 capture capacity was observed with the increase in pressure to 6 bar. The CO2 uptake was 19.8 wt% (1.34 mol CO2 per mol of IL) at 6 bar. The IL [N1,1,6,2O4][Pyrz], which is a semi-solid at room temperature, showed a lower CO2 capture capacity (7.18 wt%, 0.48 mol of CO2 per mol of IL) at 20 °C under 1 bar pressure compared to the aromatic N-heterocyclic anion based ILs. Even at higher pressure no such significant increase in the CO2 capture capacity (8.09 wt%, 0.54 mol of CO2 per mol of IL at 6 bar) was observed in the case of [N1,1,6,2O4][Pyrz].
The ILs [N1,1,6,2OH][4-Triz], [N1,1,6,2O4][4-Triz] and [N1,1,6,2OH][Imi] showed comparable capture capacity (∼1 mol of CO2 per mol of IL) at 40 °C. This revealed that chemisorption is predominant over other sorption phenomena at 40 °C (Fig. 3). The IL [N1,1,6,2O4][Pyrz] showed a relatively lower CO2 capture capacity (∼0.55 mol of CO2 per mol of IL, ∼8 wt% CO2) at 40 °C, which is due to the less thermally stable carbamate anion formed after reaction with CO2 molecules.
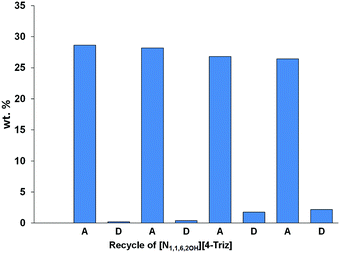
As the IL [N1,1,6,2OH][4-Triz] showed the highest CO2 capture capacity at 20 °C compared to the other studied ILs, we further studied the recyclability of this particular IL. The desorption experiments were performed using a continuous stirring under vacuum (10−3 bar) at 20 °C for 3 hours. Interestingly, no significant decrease was observed in the CO2 uptake performance even after the 4th cycle of sorption–desorption. The CO2 capture capacity was ∼27 wt% after the 4th cycle. These data suggest that the IL [N1,1,6,2OH][4-Triz] can be regenerated in an energy efficient way rather than traditional nitrogen gas purging together with heating up to 80 °C (Fig. 4).
In order to gain deeper insights into the interactions of the CO2 molecule with the IL, the [N1,1,6,2OH][4-Triz]–CO2 complex was characterized after CO2 reaction using NMR and IR spectroscopic techniques. In the 1H NMR spectrum of the IL [N1,1,6,2OH][4-Triz], an aromatic proton of the 1,2,4-triazolate anion [4-Triz] revealed a signal at 8.09 ppm before CO2 absorption, which was shifted to 8.32 ppm after CO2 absorption (see S5 in the ESI†). In the 13C NMR spectrum, the signal for aromatic carbon at 153.54 ppm is shifted to 149.85 ppm along with a new signal of carbamate at 163.88 ppm after CO2 absorption (see S11 in the ESI†). This major shift in the aromatic carbon of the 1,2,4-triazolate anion [4-Triz] is due to the lowering of aromaticity in the triazole ring after carbamate formation, which is further confirmed by DFT calculations. It could be noted that the CO2 capture capacity of [N1,1,6,2OH][4-Triz] is 28.6 wt%, 1.57 mol of CO2 per mol of IL under 1 bar pressure at room temperature (20 °C). However, theoretically the IL [N1,1,6,2OH][4-Triz] should capture 1 mol of CO2 as it is a uni-negative ion although the 1,2,4-triazolate anion [4-Triz] have three nitrogen atoms.
From the DFT calculations (Fig. 6) it is observed that the N-4 nitrogen atom is the most negatively charged (−0.31) as compared with the other two nitrogen atoms (−0.24). Thus, carbamate formation is preferred at the N-4 position, which is further confirmed by NMR having one signal in the aromatic region. The IL [N1,1,6,2OH][4-Triz] showed ∼1 equivalent CO2 capture capacity at 40 °C suggesting a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of the IL [N1,1,6,2OH][4-Triz] and a CO2 molecule. This is because physical absorption of CO2 has minor contribution to the CO2 capture capacity at 40 °C due to the simultaneous adsorption–desorption process.
1 reaction of the IL [N1,1,6,2OH][4-Triz] and a CO2 molecule. This is because physical absorption of CO2 has minor contribution to the CO2 capture capacity at 40 °C due to the simultaneous adsorption–desorption process.
The 13C solution NMR of the CO2 saturated [N1,1,6,2OH][4-Triz] IL at 20 °C showed only one new signal for carbamate formation at 163.88 ppm. There is no information regarding the physically absorbed CO2 or any other IL–CO2 complex in the solution NMR spectrum of [N1,1,6,2OH][4-Triz] after CO2 reaction. In order to obtain insights into the physically absorbed CO2, we used isotopic grade 13CO2 for our experiments. Initially, the IL [N1,1,6,2OH][4-Triz] was placed in a high pressure 5 mm Willmad NMR tube under a 13CO2 atmosphere and allowed to react with the IL for several hours. Then, the 13CO2 enriched IL sample was placed co-axially in 10 mm NMR with D2O as an external lock for the 13C NMR measurement. Interestingly, two new signals at 130.57 ppm and 158.67 ppm were observed in addition to the carbamate signal at 163.70 ppm. The new intense sharp signal at 130.57 ppm is due to the physically absorbed 13CO2, whereas the signal at 158.67 ppm is probably due to the formation of carbonic acid (–OCO2H) with the –OH group of the [N1,1,6,2OH] cation (see S12 in the ESI†).23 Surprisingly, when the 13C NMR measurement of the 13CO2 enriched sample dissolved in D2O was carried out, the signals at 130.57 ppm and 158.67 ppm disappeared again. However, after a large number of acquisitions, a small signal at 161.51 ppm was observed due to the formation of carbonate with the –OH group of the [N1,1,6,2OH] cation, which was further confirmed by the 1H–13C 2D heteronuclear multiple bond correlation (HMBC) experiment and found to be less stable in the solution form. The 1H–13C HMBC spectra are shown in the ESI† (S8) (Scheme 1).
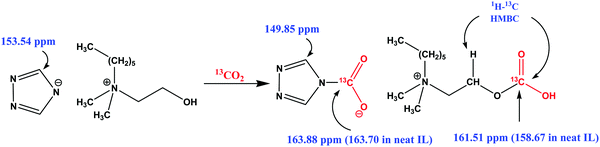 | ||
| Scheme 1 The possible reaction of the CO2 molecule with the cation and anion of the [N1,1,6,2OH][4-Triz] IL as revealed by 13C NMR spectroscopy. | ||
To further confirm these findings, FTIR measurements were carried out to characterize the IL–CO2 adducts. In the FTIR spectrum of the IL [N1,1,6,2OH][4-Triz] after CO2 reaction, three additional stretching bands at 1677 cm−1, 1635 cm−1 and a small band 2338 cm−1 were observed. The band at 1677 cm−1 is due to the formation of carbamate after CO2 reaction. The band around 1635 cm−1 is attributed to the formation of carbonic acid, which disappeared by keeping the sample under vacuum (see S12 in the ESI†). The small band at 2338 cm−1 signified the presence of physically absorbed CO2 in the IL [N1,1,6,2OH][4-Triz].24
1H NMR diffusometry was carried out to investigate the self-diffusion of ions before and after CO2 absorption. 1H NMR signals of stimulated echo for all the studied ILs before and after absorbed CO2 were observed in the whole temperature range from 20 °C to 90 °C, except for the IL [N1,1,6,2OH][Succ], for which the signal of the 1H NMR stimulated echo was observed only at temperatures equal to and higher than 40 °C. This is due to the accelerated T2 NMR relaxation of protons at lower temperatures (<40 °C) for this sample. Diffusion coefficients of anions and cations were calculated from the diffusion decays of the corresponding lines in the 1H NMR spectra. All decays were well fitted in the equation of type eqn (1). Temperature dependences of D for the cations and anions before and after CO2 adsorption are shown in Fig. 6.
A typical approach to the analysis of temperature dependence of D of ionic liquids is the Arrhenius type equation,6 which described the temperature dependence of Ds in the form
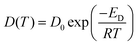 | (2) |
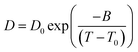 | (3) |
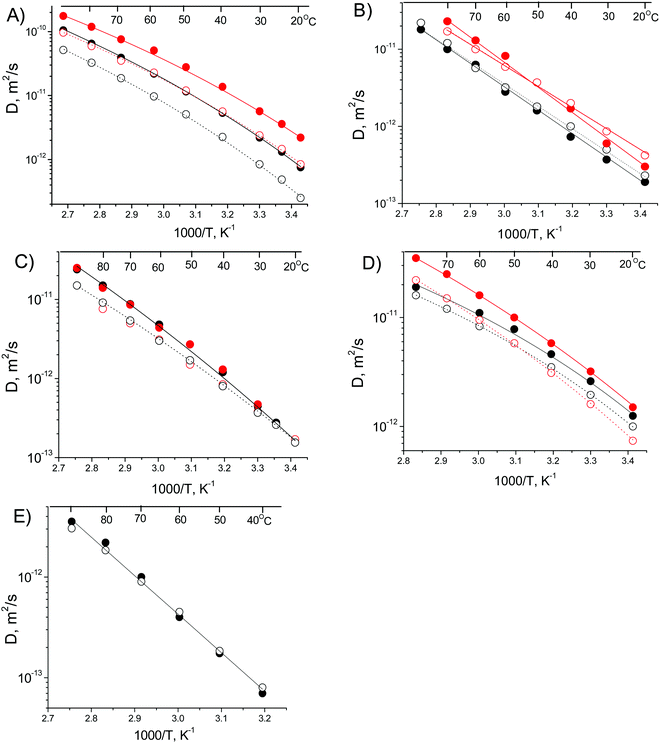 | ||
| Fig. 5 Temperature dependences of diffusion coefficients obtained from 1H PFG NMR DDs for: (A) [N1,1,6,2OH][Imi],13 (B) [N1,1,6,2O4][Pyrz], (C) [N1,1,6,2OH][4-Triz], (D) [N1,1,6,2O4][4-Triz], (E) [N1,1,6,2OH][Succ] before CO2 capture (black) and after CO2 capture (red). Solid symbols correspond to the anion and open symbols correspond to the cation. Lines correspond to the VFT equation fitting as shown in eqn (3) with the parameters of Table 1. | ||
| Ionic liquid | Ionic component | D 0, m2 s−1 | B | T 0, K | E D, kJ mol−1 K−1 |
|---|---|---|---|---|---|
| [N1,1,6,2OH][Imi] | Anion | 1.9 × 10−7 | 1510 | 170 | 12.6 ± 0.1 |
| Cation | 1.6 × 10−7 | 1620 | 170 | 13.5 ± 0.1 | |
| Anion, after CO2 absorption | 1.35 × 10−7 | 1340 | 170 | 11.1 ± 0.1 | |
| Cation, after CO2 absorption | 1.2 × 10−7 | 1440 | 170 | 12.0 ± 0.1 | |
| [N1,1,6,2OH][4-Triz] | Anion, before and after CO2 absorption | 3.5 × 10−6 | 2750 | 130 | 22.9 ± 0.1 |
| Cation, before and after CO2 absorption | 5.5 × 10−7 | 2450 | 130 | 20.4 ± 0.1 | |
| [N1,1,6,2O4][4-Triz] | Anion | 1.4 × 10−9 | 650 | 200 | 5.4 ± 0.1 |
| Cation | 1.2 × 10−9 | 660 | 200 | 5.5 ± 0.1 | |
| Anion, after CO2 absorption | 2.1 × 10−8 | 1170 | 170 | 9.7 ± 0.1 | |
| Cation, after CO2 absorption | 2.3 × 10−8 | 1270 | 170 | 10.6 ± 0.1 | |
| [N1,1,6,2O4][Pyrz] | Anion | 4.3 × 10−3 | 7000 | 0 | 58.2 ± 0.1 |
| Cation | 1.9 × 10−3 | 6700 | 0 | 55.7 ± 0.1 | |
| Anion, after CO2 absorption | 4.0 × 10−2 | 7500 | 0 | 62.3 ± 0.1 | |
| Cation, after CO2 absorption | 1.0 × 10−3 | 6300 | 0 | 52.3 ± 0.1 | |
| [N1,1,6,2OH][Succ] | Anion | 1.25 × 10−1 | 8800 | 0 | 73.2 ± 0.1 |
| Cation | 1.25 × 10−1 | 8800 | 0 | 73.2 ± 0.1 | |
From the 1H diffusometry data, we observed some general features of self-diffusion of ions before and after CO2 absorption in the studied ILs.
(1) There are two types of temperature dependences of diffusion coefficients for ILs before and after CO2 absorption: firstly, the diffusion of cations and anions is equal for [N1,1,6,2O4][Pyrz] (Fig. 5B) and [N1,1,6,2OH][Succ] (Fig. 5E). Secondly the diffusion of anions is higher than the diffusion of cations for other three ILs (Fig. 5A–D). The first type reveals weak dissociation of ions while the second type suggests higher dissociation of ions. This difference in dissociation of ions is apparently related to the form of temperature dependence of Ds. In the first type, the Arrhenius type of temperature dependence (eqn (2)) is observed while in the second case VFT type (eqn (3)) is demonstrated. From Table 1, it is seen that for the first type of temperature dependence of ILs is characteristic of low T0 and higher ED as compared with the second type of temperature dependence. Therefore, it takes higher T0 and lower ED for the dissociation of ions in the studied series of ILs. The higher viscosity of [N1,1,6,2OH][Succ] (ESI,† S10) can be related to the higher ED for this IL.
(2) At high temperature (>60 °C) the diffusion coefficients of ions for different neat ILs increase in the order [N1,1,6,2OH][Succ] < [N1,1,6,2O4][Pyrz] < [N1,1,6,2OH][4-Triz] < [N1,1,6,2O4][4-Triz] < [N1,1,6,2OH][Imi], whereas at room temperature (20 °C) the order is [N1,1,6,2OH][Succ] < [N1,1,6,2O4][Pyrz] < [N1,1,6,2OH][4-Triz] < [N1,1,6,2OH][Imi] < [N1,1,6,2O4][4-Triz], which is the same as activation energies of diffusion ED. [N1,1,6,2OH][Succ] and [N1,1,6,2O4][Pyrz] obey the Arrhenius type of temperature dependence with much higher EDs as compared with the others. Generally there is a certain relation between Ds and viscosities (ESI,† S10) in the whole series of these ILs.
(3) The diffusion coefficients of ions are increased after absorption of CO2 which is demonstrated in a higher degree for [N1,1,6,2OH][Imi], [N1,1,6,2O4][4-Triz] and [N1,1,6,2O4][Pyrz]. The absorption of CO2 also leads to the increase in ED for [N1,1,6,2O4][4-Triz] and for the anion in [N1,1,6,2O4][Pyrz], while it decreases only slightly in the case of [N1,1,6,2OH][Imi]. Generally, the physisorption and chemisorption of CO2 molecules are expected to increase the mass of the diffusion particle and thus decrease the diffusion coefficients of ions. However, the observed increase in the diffusion coefficients of ions in this case is most probably due to the decrease in the inter-ion interactions after CO2 absorption in these ILs.
13C NMR diffusometry was performed for the IL [N1,1,6,2OH][4-Triz] after absorbing 13C enriched CO2 gas in order to understand the diffusivity of both chemically reacted and physically absorbed CO2 molecules. The 13C NMR spectrum of the IL [N1,1,6,2OH][4-Triz] sample at 293 K demonstrated three intensive signals at 163 ppm, 158 ppm and 130 ppm as shown in figure S12 in the ESI.† Application of the NMR PFG technique leads to the diffusion decay of the signal amplitudes, from where diffusion coefficients were calculated using eqn (1). Signals at 163 and 158 ppm are characterized by diffusion coefficients typical for the cation and anion of [N1,1,6,2OH][4-Triz] (∼1 × 10−13 m2 s−1), while the signal at 130 ppm showed the diffusion coefficient of D = 1 × 10−7 m2 s−1. This demonstrates that the resonance line at 130 ppm belongs to the physically absorbed CO2 gas in the IL [N1,1,6,2OH][4-Triz].26
The diffusion coefficient of the physically absorbed CO2 gas is ∼106 higher than the chemically bound one (∼10−7 m2 s−1 and ∼10−13 m2 s−1, respectively at 293 K). It is known that the diffusion coefficient of CO2 at normal pressure and temperature is ∼10−5 m2 s−1.27 Although the diffusion coefficient of CO2 in the IL is less than that in the gas phase, however, the mobility of CO2 is still very high compared to other components in the IL. The diffusivity of dissolved CO2 in the IL is higher as compared with the CO2 gas dissolved in water. The diffusivity of CO2 dissolved in water is ∼10−9 m2 s−1,28 which is very close to the diffusion coefficient of water molecules. This is due to the strong network of hydrogen bonds formed in water, which incorporates CO2 molecules and products of its dissociation. In contrast, the physically dissolved CO2 does not interact that strongly with the IL. This is one of the plausible reasons that the dissolved CO2 shows a relatively high translational mobility in the IL system.
In order to further support the experimental findings, we performed NICS (Nucleus-Independent Chemical Shifts) calculations using density functional theory (DFT) to evaluate the aromaticity behavior of the N-heterocyclic anions before and after CO2 reaction. The NICS method is based on the negative of the magnetic shielding computed at the centre of the aromatic ring. A positive value implies antiaromaticity (paratropic ring current) and a negative value corresponds to aromaticity (diatropic ring current).29–33 Conventionally, NICS values are at the geometrical center (GC) of the ring denoted as NICS(0) and 1 Å above/below the perpendicular plane of the ring denoted as NICS(1). In this case due to the presence of symmetry in N-heterocyclic anions, both the ring critical point (RCP) and geometrical center (GC) coincide. According to the literature, NICS(1) (1 Å above/below the plane of the ring) is the best measure of the aromaticity descriptor than NICS(0) due to aromatic ring current and spurious contributions of the in-plane tensor components at the geometrical center of the ring. Further, the minimum effect of the local σ-bonding contributions is also observed at 1 Å above/below the molecular plane, thus, the out of plane tensor component of the NICS(1) values, NICSzz(1) shows an even better index of aromaticity.34,35
Current NICS calculations were computed through the gauge-including atomic orbital method (GIAO) implemented in GAUSSIAN 0921,36 program using the B3LYP dft functional. Due to basis set dependencies of NICS values the aug-cc-pvtz basis set was used for all NICS calculations. Ghost atoms (symbol “Bq” from Gaussian 09 input) were used as the NICS probe without using basis functions and placed 1 Å above along the line perpendicular to the molecular plane and at the center of the molecular plane.
It is observed that the NICS(0) and NICS(1) values are less influenced after carbamate formation with N-heterocyclic anions in the ILs (Table 2). Whereas NICSzz(1) values increased significantly after carbamate formation as a result of CO2 reaction with the N-heterocyclic anions of the ILs. The DFT data suggested a decrease in aromaticity of the anions after carbamate formation, which results in less pi–pi stacking interactions between the anions. The DFT optimized (gas phase) structures of 1,2,4-triazolate and the carbamate anion are shown in Fig. 6. The reduced pi–pi stacking interactions lead to faster diffusion of the anions and thus result in low viscosity of the ionic liquids upon CO2 absorption.
| Name of the anion | NICS(0) | NICS(1) | NICSZZ(1) |
|---|---|---|---|
| 4-Triazole | −11.63 | −11.70 | −36.89 |
| 4-Triazole carbamate | −11.35 | −10.70 | −31.69 |
| Pyrazole | −12.29 | −11.63 | −36.70 |
| Pyrazole carbamate | −12.21 | −10.64 | −31.33 |
| Imidazole | −11.61 | −10.74 | −35.73 |
| Imidazole carbamate | −11.18 | −9.47 | −29.27 |
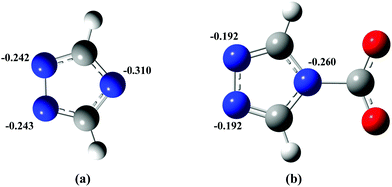 | ||
| Fig. 6 DFT optimized (gas phase) structures of (a) 1,2,4-triazolate, and (b) carbamate anion with partial charge distribution after CO2 reaction. | ||
Conclusions
Ether functionalization of a choline based cation in the IL [N1,1,6,2O4][4-Triz] resulted in lowering of viscosity due to the elimination of hydrogen bonding as compared with a non-functionalized choline based IL [N1,1,6,2OH][4-Triz]. However, CO2 capture performance as well as thermal stability was decreased significantly by ether functionalization on the cation. The IL [N1,1,6,2O4][Pyrz] was found to be a semi-solid at room temperature and slow dissociation between cations and anions in the bulk system was observed from 1H diffusion NMR data. The IL [N1,1,6,2OH][Imi] was less viscous and diffused faster as compared with the IL [N1,1,6,2OH][4-Triz] due to the presence of lesser number of hydrogen bond acceptor atoms. A significant dissociation between cations and anions was observed in both the ILs. The IL [N1,1,6,2OH][Succ] with non-aromatic N-heterocyclic anions was highly viscous and showed a lower CO2 capture capacity compared to the ILs with aromatic N-heterocyclic anions. In addition, a very slow ionic mobility with low dissociation between cations and anions in the bulk system was observed. The IL [N1,1,6,2OH][4-Triz] showed the highest CO2 capture capacity (28.6 wt%, 1.57 mol of CO2 per mol of IL, 6.48 mol of CO2 per kg of IL) at 20 °C and 1 bar pressure. in comparison with the previously reported N-heterocyclic (∼8 wt%) ILs. The high CO2 capture capacity of the IL [N1,1,6,2OH][4-Triz] is due to the contribution of the choline-based cation during the CO2 capture process by the formation of carbonic acid (–OCO2H) with the –OH group of the [N1,1,6,2OH]+ cation. The [N1,1,6,2OH][4-Triz]–CO2 adduct structure was confirmed by FTIR, 13C NMR and 1H–13C HMBC NMR experiments utilizing 13C labeled 13CO2. The recyclability performance of [N1,1,6,2OH][4-Triz] showed that the IL can be recycled under vacuum at room temperature without using any nitrogen gas and heating. It is worth noting that the ionic mobility was increased significantly after CO2 reaction in the case of ILs with aromatic N-heterocyclic anions, which can open a new area of research for low viscosity ionic liquids with promising CO2 capture performance.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The Norrbotten Research Council (NoFo) and the Swedish Research Council are gratefully acknowledged for financially supporting this project.References
- N. V. Plechkova and K. R. Seddon, Applications of ionic liquids in the chemical industry, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- J. W. Lee, J. Y. Shin, Y. S. Chun, H. B. Jang, C. E. Song and S.-G. Lee, Toward understanding the origin of positive effects of ionic liquids on catalysis: formation of more reactive catalysts and stabilization of reactive intermediates and transition states in ionic liquids, Acc. Chem. Res., 2010, 43, 985–994 CrossRef CAS PubMed.
- T. Welton, Room-temperature ionic liquids: solvents for synthesis and catalysis, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- H. Tokuda, K. Hayamizu, K. Ishii, Md. A. B. Hasan Susan and M. Watanabe, Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation, J. Phys. Chem. B, 2005, 109, 6103–6110 CrossRef CAS PubMed.
- H. Tokuda, S. Tsuzuki, Md. A. B. Hasan Susan, K. Hayamizu and M. Watanabe, How ionic are room-temperature ionic liquids? An indicator of the physicochemical properties, J. Phys. Chem. B, 2006, 110, 19593–19600 CrossRef CAS PubMed.
- P. Atkins and J. de Paula, Atkin's Physical Chemistry, Oxford University Press, 10th edn, 2014, p. 1008 Search PubMed.
- T. L. Greaves and C. J. Drummond, Protic ionic liquids: evolving structure–property relationships and expanding applications, Chem. Rev., 2015, 115, 11379–11448 CrossRef CAS PubMed.
- K. Damodaran, Recent NMR studies of ionic liquids, Annu. Rep. NMR Spectrosc., 2016, 88, 215–244 CrossRef CAS.
- R. Hayes, G. G. Warr and R. Atkin, Structure and nanostructure in ionic liquids, Chem. Rev., 2015, 115, 6357–6426 CrossRef CAS PubMed.
- A. E. Frise, T. Ichikawa, M. Yoshio, H. Ohno, S. V. Dvinskikh, T. Kato and I. N. Furo, Ion conductive behaviour in a confined nanostructure: NMR observation of self-diffusion in a liquid-crystalline bicontinuous cubic phase, Chem. Commun., 2010, 46, 728–730 RSC.
- A. Filippov, F. U. Shah, M. Taher, S. Glavatskih and O. N. Antzutkin, NMR self-diffusion study of a phosphonium bis(mandelato)borate ionic liquid, Phys. Chem. Chem. Phys., 2013, 15, 9281–9287 RSC.
- A. Filippov, M. Taher, F. U. Shah, S. Glavatskih and O. N. Antzutkin, Effect of length of long alkyl chains of cations on diffusion and density in pyrrolidinium bis(mandelato)borate ionic liquids, Phys. Chem. Chem. Phys., 2014, 16, 26798–26805 RSC.
- S. Bhattacharyya, A. Filippov and F. U. Shah, Insight into the effect of CO2 absorption on the ionic mobility of ionic liquids, Phys. Chem. Chem. Phys., 2016, 18, 28617–28652 RSC.
- P. T. Callaghan, Principles of Nuclear Magnetic Resonance Microscopy, Clarendon, Oxford, 1991 Search PubMed.
- M. Ramdin, T. W. de Loos and T. J. H. Vlugt, State-of-the-art of CO2 capture with ionic liquids, Ind. Eng. Chem. Res., 2012, 51, 8149–8177 CrossRef CAS.
- S. Bhattacharyya and F. U. Shah, Ether functionalized choline tethered amino acid ionic liquids for enhanced CO2 capture, ACS Sustainable Chem. Eng., 2016, 4, 5441–5449 CrossRef CAS.
- M. Petkovic, J. L. Ferguson, H. Q. N. Gunaratne, R. Ferreira, M. C. Leitao, K. R. Seddon, L. P. N. Rebeloa and C. S. Pereira, Novel biocompatible cholinium-based ionic liquids: toxicity and biodegradability, Green Chem., 2010, 12, 643–649 RSC.
- J. I. Santos, A. M. M. Goncalves, J. L. Pereira, B. F. H. T. Figueiredo, F. A. de Silva, J. A. P. Coutinho, S. P. M. Venturab and F. Goncalvesa, Environmental safety of cholinium-based ionic liquids: assessing structure–ecotoxicity relationships, Green Chem., 2015, 17, 4657–4668 RSC.
- X.-D. Hou, Q.-P. Liu, T. J. Smith, N. Li and M.-H. Zong, Evaluation of Toxicity and Biodegradability of Cholinium Amino Acids Ionic Liquids, PLoS One, 2013, 8, e59149 Search PubMed.
- J. E. Tanner, Use of the stimulated echo in NMR diffusion studies, J. Chem. Phys., 1970, 52, 2523–2526 CrossRef CAS.
- M. J. Frisch et al. , Gaussian 09, Revision A. 01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- S. Seo, M. Q. Guzman, M. A. DeSilva, T. B. Lee, Y. Huang, B. F. Goodrich, W. F. Schneider and J. F. Brennecke, Chemically Tunable Ionic Liquids with Aprotic Heterocyclic Anion (AHA) for CO2 Capture, J. Phys. Chem. B, 2014, 118, 5740–5751 CrossRef CAS PubMed.
- C. Chatterjee and A. Sen, Sensitive colorimetric sensors for visual detection of carbon dioxide and sulfur dioxide, J. Mater. Chem. A, 2015, 3, 5642–5647 CAS.
- T. Seki, J.-D. Grunwaldt and A. Baiker, In Situ Attenuated Total Reflection Infrared Spectroscopy of Imidazolium-Based Room-Temperature Ionic Liquids under “Supercritical” CO2, J. Phys. Chem. B, 2009, 113, 114–122 CrossRef CAS PubMed.
- A. Noda, K. Hayamizu and M. Watanabe, Pulsed-gradient spin-echo 1H and 19F ionic diffusion coefficient, viscosity, and ionic conductivity of non-chloroaluminate room-temperature ionic liquids, J. Phys. Chem. B, 2001, 105, 4603–4610 CrossRef CAS.
- H. Omi, T. Ueda, K. Miyakubo and T. Eguchi, Dynamics of CO2 molecules confined in the micropores of solids as studied by 13C NMR, Appl. Surf. Sci., 2005, 252, 660–667 CrossRef CAS.
- E. R. S. Winter, Diffusion properties of gases. Part IV. The self-diffusion coefficients of nitrogen, oxygen and carbon dioxide, J. Chem. Soc., 1950, 1170, 342–347 Search PubMed.
- R. E. Zeebe, On the molecular diffusion coefficients of dissolved CO2, HCO3−, and CO32− and their dependence on isotopic mass, Geochim. Cosmochim. Acta, 2011, 75, 2483–2498 CrossRef CAS.
- P. Lazzereti, in Prog. Nucl. Magn. Reson. Spectrosc., ed. J. W. Emsley, J. Feeney and L. H. Sutcliffe, Elsevier, Amsterdam, 2000, vol. 36, p. 1 Search PubMed.
- P. Lazzeretti, Assessment of aromaticity via molecular response properties, Phys. Chem. Chem. Phys., 2004, 6, 217–223 RSC.
- J. Aihara, Nucleus-independent chemical shifts and local aromaticities in large polycyclic aromatic hydrocarbons, Chem. Phys. Lett., 2002, 365, 34–39 CrossRef CAS.
- N. H. Martin, D. M. Loveless, K. L. Main and D. C. Wade, Computation of through-space NMR shielding effects by small-ring aromatic and antiaromatic hydrocarbons, J. Mol. Graphics Modell., 2006, 25, 389–395 CrossRef CAS PubMed.
- P. v. R. Schleyer, C. Maerker, A. Dransfield, H. Jiao and N. J. R. van Eikema Hommes, Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS PubMed.
- P. v. R. Scheleyer, M. Manoharan, Z. X. Wang, B. Kiran, H. J. Jiao, R. Puchta and N. J. R. v. E. Hommes, Dissected Nucleus-Independent Chemical Shift Analysis of π-Aromaticity and Antiaromaticity, Org. Lett., 2001, 3, 2465–2468 CrossRef.
- C. Corminboeuf, T. Heine, G. Seifert, P. v. R. Schleyer and J. Weber, Induced magnetic fields in aromatic [n]-annulenes—interpretation of NICS tensor components, Phys. Chem. Chem. Phys., 2004, 6, 273–276 RSC.
- K. Wolinski, J. F. Hilton and P. Pulay, Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations, J. Am. Chem. Soc., 1990, 112, 8251–8260 CrossRef CAS.
- A. Filippov, N. Azancheev, A. Gibaydullin, S. Bhattacharyya, O. N. Antzutkin and F. U. Shah, Dynamic properties of imidazolium orthoborate ionic liquids mixed with polyethylene glycol studied by NMR diffusometry and impedance spectroscopy, Magn. Reson. Chem., 2017 DOI:10.1002/mrc.4636.
- C. Wang, X. Luo, H. Luo, D. Jiang, H. Li and S. Dai, Tuning the basicity of ionic liquids for equimolar CO2 capture, Angew. Chem., Int. Ed., 2011, 50, 4918–4922 CrossRef CAS PubMed.
- S. Seo, M. Q. Guzman, M. A. DeSilva, T. B. Lee, Y. Huang, B. F. Goodrich, W. F. Schneider and J. F. Brennecke, Chemically tunable ionic liquids with aprotic heterocyclic anion (AHA) for CO2 capture, J. Phys. Chem. B, 2014, 118, 5740–5751 CrossRef CAS PubMed.
- X. Zhu, M. Song and Y. Xu, DBU-based protic ionic liquids for CO2 capture, ACS Sustainable Chem. Eng., 2017, 5, 8192–8198 CrossRef CAS.
- C. Wang, H. Luo, D. Jiang, H. Li and S. Dai, Carbon dioxide capture by superbase-derived protic ionic liquids, Angew. Chem., Int. Ed., 2010, 49, 5978–5981 CrossRef CAS PubMed.
- R. Vijayraghavan, S. J. Pas, E. I. Izgorodina and D. R. MacFarlane, Diamino protic ionic liquids for CO2 capture, Phys. Chem. Chem. Phys., 2013, 15, 19994–19999 RSC.
- M. H. Cohen and D. Turnbull, Molecular transport in liquids and gases, J. Chem. Phys., 1959, 31, 1164–1169 CrossRef CAS.
- F. U. Shah, O. I. Gnezdilov and A. Filippov, Ion dynamics in halogen-free phosphonium bis(salicylato)borate ionic liquid electrolytes for lithium-ion batteries, Phys. Chem. Chem. Phys., 2017, 19, 16721–16730 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cp07059d |
| This journal is © the Owner Societies 2017 |