Two-dimensional structural ordering in a chromophoric ionic liquid for triplet energy migration-based photon upconversion†
Abstract
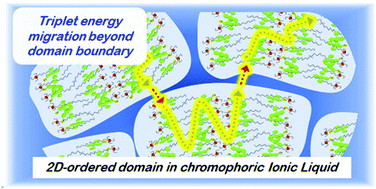
A novel chromophoric ionic liquid (IL) with two-dimensional (2D) nanostructural order is developed, and its structure–property relationship is investigated by harnessing photon upconversion based on triplet energy migration. An ion pair of 9,10-diphenylanthracene-2-sulphonate (DPAS) and asymmetric quaternary phosphonium ion exhibited both ionic crystal (IC) and supercooled IL phases at room temperature. Single crystal X-ray analysis of the IC phase showed an alternate alignment of polar (ionic) and non-polar (non-ionic) layers, and this layered structure was basically maintained even in the IL phase. The diffusion length of triplet excitons in the IL phase, obtained by the analysis of upconverted emission in succession to triplet–triplet annihilation (TTA), is larger than the domain size estimated from powder X-ray analysis. This suggests that triplet excitons in chromophoric ILs can diffuse over the nanostructured domains.
- This article is part of the themed collection: Complex molecular systems: supramolecules, biomolecules and interfaces


 Please wait while we load your content...
Please wait while we load your content...