Curly arrows, electron flow, and reaction mechanisms from the perspective of the bonding evolution theory
Abstract
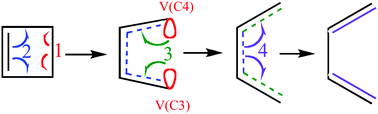
Despite the usefulness of curly arrows in chemistry, their relationship with real electron density flows is still imprecise, and even their direct connection to quantum chemistry is still controversial. The paradigmatic description – from first principles – of the mechanistic aspects of a given chemical process is based mainly on the relative energies and geometrical changes at the stationary points of the potential energy surface along the reaction pathway; however, it is not sufficient to describe chemical systems in terms of bonding aspects. Probing the electron density distribution during a chemical reaction can provide important insights, enabling us to understand and control chemical reactions. This aim has required an extension of the relationships between the concepts of traditional chemistry and those of quantum mechanics. Bonding evolution theory (BET), which combines the topological analysis of the electron localization function (ELF) and Thom's catastrophe theory (CT), provides a powerful method that offers insight into the molecular mechanism of chemical rearrangements. In agreement with the laws of physical and aspects of quantum theory, BET can be considered an appropriate tool to tackle chemical reactivity with a wide range of possible applications. In this work, BET is applied to address a long-standing problem: the ability to monitor the flow of electron density. BET analysis shows a connection between quantum mechanics and bond making/forming processes. Likewise, the present approach retrieves the classical curly arrows used to describe the rearrangements of chemical bonds and provides detailed physical grounds for this type of representation. We demonstrate this procedure using the test set of prototypical examples of thermal ring apertures, and the degenerated Cope rearrangement of semibullvalene.
- This article is part of the themed collections: PCCP Perspectives and 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...