Analysis of dispersive interactions at polymer/TiAlN interfaces by means of dynamic force spectroscopy†
M.
Wiesing
 a,
T.
de los Arcos
a,
M.
Gebhard
a,
T.
de los Arcos
a,
M.
Gebhard
 b,
A.
Devi
b,
A.
Devi
 b and
G.
Grundmeier
*a
b and
G.
Grundmeier
*a
aTechnical and Macromolecular Chemistry, University of Paderborn, Warburger Straße 100, 33098 Paderborn, Germany. E-mail: g.grundmeier@tc.uni-paderborn.de
bInorganic Chemistry II, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
First published on 21st November 2017
Abstract
The structural and electronic origins of the interactions between polycarbonate and sputter deposited TiAlN were analysed using a combined electron and force spectroscopic approach. Interaction forces were measured by means of dynamic force spectroscopy and the surface polarizability was analysed by X-ray photoelectron valence band spectroscopy. It could be shown that the adhesive interactions between polycarbonate and TiAlN are governed by van der Waals forces. Different surface cleansing and oxidizing treatments were investigated and the effect of the surface chemistry on the force interactions was analysed. Intense surface oxidation resulted in a decreased adhesion force by a factor of two due to the formation of a 2 nm thick Ti0.21Al0.45O surface oxide layer. The origin of the residual adhesion forces caused by the mixed Ti0.21Al0.45O surface oxide was clarified by considering the non-retarded Hamaker coefficients as calculated by Lifshitz theory, based on optical data from Reflection Electron Energy Loss Spectroscopy. This disclosed increased dispersion forces of Ti0.21Al0.45O due to the presence of Ti(IV) ions and related Ti 3d band optical transitions.
Introduction
The thermal processing of polymer melts is affected by different interfacial phenomena such as interfacial polymer degradation, corrosion and polymer adhesion to tool surfaces.1 Especially in the case of polymers used for optical applications such as polycarbonate (PC), these phenomena deteriorate the stability of the process and the quality of the resulting product due to strong adhesion of the polymer to the steel surfaces.2 In this regard, the adhesion of polycarbonate is decreased by the application of nitridic hard coatings such as TiAlN, resulting in a reduction of the demoulding forces.2 However, PC can still adhere strongly to TiAlN, leading in the case of coated screws to the formation of strongly adhering degradation layers, which form discoloured speckles in extruded films by shear induced release.1,2 These phenomena illustrate that the adhesive properties of TiAlN hard coatings for plastic processing applications are of high relevance for the performance of coated tools and a more detailed understanding of the origin of the adhesive interactions is required. This aspect is therefore approached in this work by investigating the adhesive force interactions of TiAlN hard coatings in contact with polycarbonate.An important factor for understanding adhesion is the surface chemistry of the interface. For the case of the TiAlN surface earlier studies based on X-ray Photoelectron Spectroscopy (XPS) showed that the residual oxygen in the reactor and the ambient atmosphere results in surface oxidation and the formation of a Ti(IV) containing cover layer.3 Furthermore, sputter depth profiling of TiAlN hard coatings after deposition and exposure to atmosphere disclosed that the surface oxidation of TiAlN includes surface enrichment of aluminium and is accompanied by reactive inward migration of oxygen and the formation of an buried oxynitride growth region.4,5 This principal oxidation mechanism was further validated by investigating the early oxidation of TiAlN at reduced pressures of 10−6 Pa and at room temperature.6,7 Notably, the chemisorption of oxygen on the Ar+ sputter cleaned TiAlN surface was shown to proceed selectively at superficial Ti atoms due to the interaction with the Ti 3d spill-over electrons. An oxynitride growth region and ultimately a Ti(IV)-containing top layer then develops at increasing pressures. It could be shown that the Ti(IV)-containing top layer was nitrogen doped and segregated when formed at room temperature but was mixed at an atomic scale when oxidizing at elevated temperatures.
In the work presented here, Dynamic Force Spectroscopy (DFS) is used to probe the complex force interactions between TiAlN coated tips and thin polycarbonate films. DFS was developed recently as a routine method in Ultra High Vacuum Atomic Force Microscopy (UHV-AFM) studies for the investigation interaction forces.8–11 This method is based on the measurement of the frequency shift of a vibrating cantilever induced by the tip–sample forces. The frequency shift can then be deconvoluted to obtain the tip–sample force.12,13 The key benefit of this method is the stability of the vibrating cantilever, which gives access to the near-contact regime, where soft cantilevers become unstable and snap onto the surface due to the tip–sample forces.12 Based on the continuity of the determined force curve, this approach allows to distinguish between individual force contributions according to their different decay lengths.14 Furthermore, three dimensional potential volumes can be probed with pico-newton sensitivity, which is of high relevance for understanding not only atomic scale adhesion but also friction.11,15 So far, DFS has not widely been used for studying adhesion to polymeric surfaces and only its general applicability has been demonstrated.16
The influence of the surface oxidation of a hard coating on adhesion was investigated earlier for the case of amorphous AlYB14 in contact with polyethylene using ab initio simulation and UHV atomic force spectroscopy.17 It was found that the loss of metallic conductivity of AlYB14 upon oxidation increased the adhesion of the polyethylene due to dominating force contributions by dipolar interactions. In addition, the relevance of chemical binding contributions for adhesion has been highlighted for the case of polypropylene and TiAlN.18Ab initio calculations indicated that the work of separation of polypropylene was largely affected by abstraction of hydrogen atoms or by chain scission, which increase the work of separation by two orders of magnitude. These examples illustrate the complexity of the interactions between hard coatings and polymers. Thus, the forces between PC and TiAlN hard coatings are herein investigated regarding the effect of different surface chemical compositions and structures: TiAlN covered by a complex multi-layered oxide as a result of exposure to ambient atmosphere after deposition and TiAlN after intentional strong surface oxidation.
The results section first presents the chemical properties of the investigated surfaces as they critically affect the adhesion forces. The interaction forces between TiAlN and polycarbonate as determined by DFS are then discussed on the basis of the varying substrate surface chemistries. Finally, the detailed discussion of the electronic origin of the adhesion is supported by comparison with reference materials, valence band spectroscopy and the calculation of Hamaker constants.
Experimental
Thin film deposition
TiAlN and TiAl(O,N) hard coatings were deposited by High Power Pulsed Magnetron Sputtering (HPPMS) using a CemeCon CC-800/9 at the Materials Chemistry division of the RWTH Aachen.3 A rectangular magnetron with a Ti0.5Al0.5 compound target was used. The p-Si(100) wafer (1–5 Ω cm) and cantilever (NSC15, Mikromasch) substrates were mounted at a target-to-substrate distance of 80 mm and were neither heated nor cooled. The base pressure was below 0.2 mPa and the gas flows during deposition were 200 sccm Ar and 50 sccm N2 for TiAlN and 200 sccm Ar, 50 sccm N2 and 7 sccm O2 for TiAl(O,N) resulting in partial pressures of 358 mPa, 91 mPa and 9 mPa, respectively. A Melec HPPMS generator with a frequency of 800 Hz, a duty cycle of 4% and a time-averaged power of 3000 W resulting in peak power densities of around 450 W cm−2 was used. Thin film deposition was carried out at floating potential. The films deposited on Si wafer were then used for surface chemical analysis by XPS.The bulk compositions of the coatings were determined by XPS after removal of the surface oxidation layers by Ar+ ion sputtering (3 kV, 30 μA cm−2, 5 min). Due to preferential sputtering of especially the light elements, these values only represent estimates for the stoichiometries of the original films.4,19 The bulk stoichiometries shown in Table 1 revealed the presence of around 7 at% oxygen in the TiAlN film presumably as a result of oxygen contaminations present in the chamber during deposition.
| Coating | O 1s/at% | N 1s/at% | Ti 2p/at% | Al 2p/at% | Ti/Al |
|---|---|---|---|---|---|
| TiAlN | 7.6 | 40.0 | 21.2 | 31.2 | 0.67 |
| TiAl(O,N) | 30.2 | 23.5 | 18.8 | 26.8 | 0.70 |
As reference materials, TiO2 and Al2O3 thin films of 45 nm thickness were deposited at the Chair II of Inorganic Chemistry at the Ruhr University Bochum on Si-wafer substrates and cantilevers by means of Plasma Enhanced Atomic Layer Deposition (PEALD) employing tetrakis(dimethylamido)-titanium(IV) (TDMAT) and trimethyl-aluminum (TMA) as precursors. TDMAT was synthesized according to literature and was found to be spectroscopically pure (1H-NMR), while TMA was purchased by Strem Chemicals.20 All depositions were carried out in the reactor chamber described elsewhere; in the case of TiO2 an earlier optimized process with a growth rate of 0.92 Å per cycle was adopted.21 The deposition process for Al2O3 was as follows: TMA (STREM, min. 98%) was kept in a stainless steel container at constantly 273 K. The precursor was pulsed for 13 ms followed by Ar-purging (25 sccm, 99.999%, Alfa gas) for 950 ms to remove excess of precursor. After a total of 1000 ms, O2 was introduced over a time of 500 ms (25 sccm, 99.995%, Alfa gas) while the plasma ignition took place 100 ms after starting the O2 flow. The plasma step took 200 ms and was again followed by an Ar-purge step to remove reaction by-products and excess of O2. For all depositions, the substrate holder and reactor chamber were heated to 373 and 333 K, respectively. The growth rate for this Al2O3-PEALD process was found to be 1.4 Å per cycle. The TiO2 and Al2O3 PEALD thin films were X-ray amorphous.
A thin plasma polymer film based on hexamethyldisiloxane (PP-HMDSO) was used as reference material with a low surface energy and was deposited on flat Si wafer using a home-built radio frequency reactor. The total pressure during deposition was 30 Pa and the Ar/HMDSO ratio was 6.
The voltage during deposition was 440 V and the power density 0.03 W cm−2. The film was analysed by FTIR and XPS and the results can be found in the ESI.† In essence, the PP-HMDSO is composed of Si–H, Si–CHx and Si–O–Si groups.
Polymer thin films of polystyrene and polycarbonate were deposited by spin-coating onto Si wafer substrates using 2 wt% polycarbonate/1,4-dioxane and 2 wt% polystyrene/toluol solutions (room temperature, 2000 rpm). All chemicals were of analytical grade and the bisphenol-A polycarbonate (Makrolon 3108, Goodfellow) as well as the polystyrene (193![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mw, Aldrich) were free of additives. The thickness of the thin films was around 60 nm as determined by spectroscopic ellipsometry and the use of tabulated optical constants (PP-HMDSO was approximated by polydimethyldisiloxane (PDMS)).
000 Mw, Aldrich) were free of additives. The thickness of the thin films was around 60 nm as determined by spectroscopic ellipsometry and the use of tabulated optical constants (PP-HMDSO was approximated by polydimethyldisiloxane (PDMS)).
Electron spectroscopy
X-ray photoelectron spectroscopy was performed on the flat samples using an ESCA+ system (ScientaOmicron) at a base pressure of <1 × 10−8 Pa. Monochromatized Al Kα irradiation (1486.7 eV) was used for excitation resulting in an energy resolution of 0.77 eV for high resolution and 2.2 eV for wide-range survey spectra as determined from the FWHM of the Ag 3d5/2 line. The angle of emission was 60° relative to the surface normal resulting into a depth of information of 2.8 nm.22 The binding energy scale was referenced to the binding energy of Ag 3d5/2 at 368.0 eV. Data evaluation was performed using CasaXPS (v2.3.16). Reflection Electron Energy Loss Spectroscopy (REELS) was measured using an electron beam (Nanofocus 50, Staib Instruments) at an energy of 450 eV for polycarbonate and the O beam oxidized TiAlN and at an energy of 1050 eV for the Al2O3 and TiO2 PEALD thin films. (In the case of the PEALD films the surfaces were cleaned from organic contaminations using the surface cleansing procedure described below.) The energy resolution was 1.0 eV and the angles of incidence and emission were both 30°. The beam was rastered across an area of 0.07 mm2 resulting into a current density of 0.44 μA cm−2. In order to prevent beam damage, the polycarbonate sample was continuously moved during the measurement limiting the dose to 7.1 μC cm−2, where no beam damage was observed.23Ion beam treatments
Cantilevers were cleansed before use (“surface cleansing”) by means of an oxygen beam at 0 eV acceleration voltage for 7 min (GenII, Tectra, Germany). The source was operated in the downstream mode so that the ions were largely thermalized and no net current was observed when biasing the sample to 3.3 V. A more detailed characterization of the source can be found in literature.24,25 The UHV system allowed the transfer of the surface-cleansed cantilevers into the AFM while maintaining a pressure of below 1 × 10−7 Pa. Intense oxidation of the TiAlN surface (“O beam oxidation”) was achieved by irradiating the sample with an O beam accelerated by 1.5 kV for 2 min at normal incidence with a flux of around 40 μA cm−2. During operation the background pressure of the chamber was around 5 × 10−2 Pa. For XPS valence band spectroscopy and bulk stoichiometric analysis the surface oxides present on the TiAlN and TiAl(O,N) thin films were removed by Ar+ sputtering at 3 keV and a flux of 30 μA cm−2 for 5 min (Fig05 Ion Gun, Physical Electronics, USA) until the oxygen concentration as determined by XPS was constant.UHV-AFM
Dynamic Force Spectroscopy (DFS) was measured using a variable temperature AFM (ScientaOmicron) directly attached to the ESCA+ system at a base pressure of <2 × 10−8 Pa. Force spectroscopy was performed in constant amplitude mode using the coated cantilevers with q factors of around 3500–4000 mounted on adhesive carbon pads. The frequency shift curves were measured with 25 pm height resolution and a dwell time of 40–60 ms. Around 20 curves were acquired at the same position and averaged for data evaluation. The individual spring constants of the cantilevers were calibrated using the Sader method.26,27 The amplitude was calibrated using the constant frequency shift method with increasing amplitudes until the sensitivity was constant (typically 45–50 mV nm−1).28 The frequency shifts were transformed to interaction potentials based on the theoretical approach of Sader and Jarvis.13Results and discussion
Surface chemical characterization by XPS
For DFS measurements the cantilevers coated with the TiAlN hard coating were used after removal of adventitious organic contaminations by means of oxygen beam irradiation at 0 V acceleration voltage for 7 min (“surface cleansing”). The stoichiometry of the TiAlN surface before and after surface cleansing is shown in Table 2 and it was observed that the carbon concentration as derived from the C 1s signal was decreased from 25.5 at% to 1.2 at%. The surface cleansing therefore effectively removes the superficial organic contaminations. Concomitantly, the Ti/Al ratio increased from 0.35 to 0.40. This finding is most probably not explained by outward migration of Ti but rather by the loss of the organic contamination layer, which screens the slower Ti 2p photoelectrons more effectively than the faster Al 2p electrons.29 Further, when considering that the bulk Ti/Al ratio is around 0.7 (Table 1), it follows that Al is enriched at the oxidized surface due to outward migration, which is typical for TiAlN hard coatings.4 The surface cleansing procedure was also applied to the TiAl(O,N), TiO2 and Al2O3 coated cantilevers and similarly the amount of organic contaminations could be decreased to below 1.0 at% (see Table 2).| Coating | Treatment | C 1s/at% | O 1s/at% | N 1s/at% | Ti 2p/at% | Al 2p/at% | Ti/Al |
|---|---|---|---|---|---|---|---|
| TiAlN | Atmosphere exposed | 25.5 | 31.1 | 15.3 | 7.2 | 20.8 | 0.35 |
| Surface cleansed | 1.2 | 38.7 | 20.2 | 11.4 | 28.5 | 0.40 | |
| O beam oxidized | 0.4 | 55.2 | 8.0 | 11.8 | 24.6 | 0.48 | |
| TiAl(O,N) | Surface cleansed | 0.8 | 50.0 | 11.0 | 13.0 | 25.2 | 0.52 |
| TiO2 | Surface cleansed | 0.5 | 71.3 | — | 28.2 | — | — |
| Al2O3 | Surface cleansed | 0.3 | 58.2 | — | — | 41.5 | — |
For investigating the effect of enhanced surface oxidation on the interaction forces the TiAlN coated cantilevers were oxidized by O beam irradiation at 1.5 kV acceleration voltage for 2 min (“O beam oxidation”). As shown in Table 2, the concentration of oxygen increased after O beam oxidation from 31.1 at% to 55.2 at% indicating intense surface oxidation. The Ti/Al ratio also slightly increased from 0.40 to 0.48 as a result most probably of sputtering damage.30
A more detailed characterization of the surface cleansed and O beam oxidized samples was performed by high-resolution XPS. For the sake of clarity, the discussion is limited to the Ti 2p spectrum but a detailed discussion of the N 1s, O 1s and Al 2p core level spectra can be found in the ESI.†
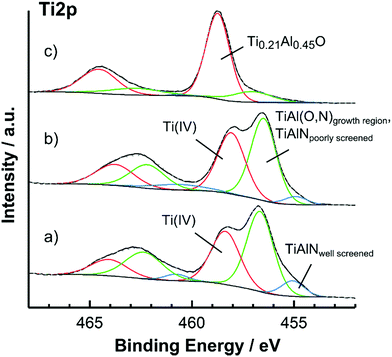
As shown in Fig. 1a and b, the Ti 2p spectra of the atmosphere exposed and surface cleansed TiAlN surface could be mathematically described by three components located at 455.1, around 456.7 and at 458.3 eV. These peaks are ascribed to the well screened component of non-oxidized TiAlN (455.1 eV), to the oxynitridic TiAl(O,N) growth region and the poorly screened component of non-oxidized TiAlN (both at 456.7 eV) and to a surface oxide with Ti being in the +IV state (458.3 eV). The peak separation due to spin–orbit coupling was fixed to 5.7 eV. The overall line shape is complicated by many-body processes as well as shake-ups and is discussed in detail elsewhere.7,31–33 Among these effects, it is noted that in the TiAlN substrate the Ti 2p core hole is supposed to be only partially screened by free d-electrons during the photoemission process.31,34 In this regards, electrostatic interactions between the core hole and the valence band pull a d-state down to below the Femi level. This state can then be filled by a d-electron resulting into a relaxed state and a Ti 2p signal at lower binding energies (“well screened” component) or can be left unoccupied leading to a higher binding energy “poorly screened” component. In accordance to previous reports, the poorly screened component is expected around 457 eV and should overlap with the TiAl(O,N) signal.31,32 This shows that different spectral components not necessarily reflect different chemical species.
The components present in the spectrum indicate a complex multi-layered surface oxide which can be described from the bulk to the surface as TiAlN|TiAl(O,N)|(Ti(IV),Al) oxide in agreement with literature.5,35 It is noted that the oxynitridic TiAl(O,N) layer resembles the growth region of the surface oxide and develops due to the reactive inward migration of oxygen.7 The reactive inward migration mechanism also implies most probably the existence of composition gradients and smooth transitions between the different surface phases.
After surface cleansing, the spectrum in Fig. 1b shows an increase of the Ti(IV) component from 36.8% to 42.3% of the total Ti 2p peak area indicating that the surface was slightly oxidized. As deduced from the fraction of the Ti(IV) and TiAl(O,N) components and by using an effective attenuation length of 1.9 nm, the thicknesses of the Ti(IV) and the TiAl(O,N) layers were 0.5 and 1.8 nm.22,29 It is noted that the thickness values herein reported were calculated based on a surface layer model with sharp interfaces, which is not necessarily the case as mentioned above. However, despite the possible presence of related systematic errors such thickness values are supposed to reflect the spatial expansion of layers even in the case of smooth boundaries according to literature.29 Apart from the slight increase of the Ti(IV) component by around 5% and the effective removal of carbon the original surface chemistry was not affected by the surface cleansing procedure and this clearly shows that the cleansed TiAlN cantilevers adequately resemble the surface chemistry of the hard coating.
In contrast, the line shape of the TiAlN surface after O beam oxidation was governed by the presence of the Ti(IV) component at 458.7 eV, which was increased at the expense of the TiAl(O,N) component at 457.1 eV. Further, the component of the non-oxidized TiAlN substrate at 455.1 eV was completely absent. According to its binding energy, the Ti(IV) component might either relate to segregated TiO2 or mixed TiAlO oxide. This situation was clarified by the O 1s modified Auger parameter, which was determined to be 1040.6 ± 0.1 eV and indicated by comparison with literature the presence of mixed TiAlO.36 In contrast, the precise composition of the TiAlO at the surface is less clear due to the surface enrichment of Al. However, the composition of this layer can be estimated from the Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratios obtained by XPS (see Table 2) to be Ti0.21Al0.45O. The thickness of the Ti0.21Al0.45O top layer was around 2.0 nm and was increased by O beam oxidation as compared with the thickness of the Ti(IV) top layer of the surface cleansed TiAlN coating. Again it is noted that most probably smooth layer boundaries are present due to atomic mixing during the oxygen bombardment.30 However, the surface oxide layer structure after O beam oxidation is formally described as TiAlN|TiAl(O,N)|Ti0.21Al0.45O.
O ratios obtained by XPS (see Table 2) to be Ti0.21Al0.45O. The thickness of the Ti0.21Al0.45O top layer was around 2.0 nm and was increased by O beam oxidation as compared with the thickness of the Ti(IV) top layer of the surface cleansed TiAlN coating. Again it is noted that most probably smooth layer boundaries are present due to atomic mixing during the oxygen bombardment.30 However, the surface oxide layer structure after O beam oxidation is formally described as TiAlN|TiAl(O,N)|Ti0.21Al0.45O.
The spectra of the atmosphere exposed and surface cleansed TiAl(O,N) films were similar to the case of TiAlN as described above and the same surface oxide layering was observed with the only difference that after surface cleansing the Ti(IV) layer was increased to 0.6 nm and the TiAl(O,N) layer to 2.6 nm thickness. The corresponding spectra can be found in the ESI.†
Contact formation: processes and structures formed during DFS
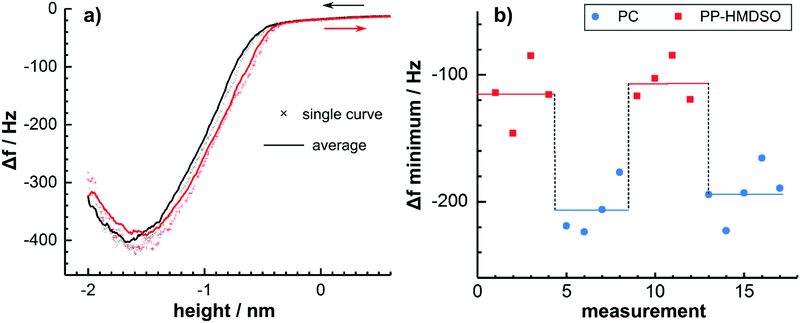
The interaction forces between the surface cleansed TiAlN and the underlying PC as well as an HMDSO plasma polymer (PP-HMDSO) were analysed by means of DFS. The spectroscopic and topographic analysis of the thin polymer films is shown in the ESI.†Fig. 2a shows a single frequency shift Δf curve in comparison with an average from subsequently measured curves. It was observed that the frequency shift during approach and retraction of the tip were basically identical but slightly shifted on the height axis, presumably as a result of piezo hysteresis. Furthermore, when measuring repeatedly at the same spot, no changes of the frequency shift were observed and the contact could be considered as mechanically stable. This fundamental observation shows that the measurement between TiAlN and PC is reversible and reproducible. Consequently, multiple curves were measured and averaged for data evaluation.
It is worth mentioning that the frequency shift did not show discontinuities, which are uniquely related to hysteretic forces.37 This result is counterintuitive, because the polymeric substrate is expected to relax by the formation of a neck accompanied by a hysteresis in the approach and retract curve.37,38 This finding is explained when considering that the measured frequency shift is time averaged and that binding and unbinding are statistical processes.37 However, the shape of the Δf curve is further complicated by the formation of single-molecular polymer bridges between tip and sample as discussed below.
Fig. 2b shows minima of averaged frequency shift curves measured on different sample locations alternating on PC and the PP-HMDSO film. PP-HMDSO was studied as a highly cross-linked reference polymer with a low surface energy in analogy to the chemically related polydimethylsiloxane (PDMS). It was observed that the frequency shift minimum reversibly switches when changing the substrate from around −120 Hz in the case of the PP-HMDSO to around −200 Hz when measuring on PC. Consequently, it can be concluded that the unbinding of the TiAlN tip from the polymer substrate occurs reversibly and no material is transferred to the tip.
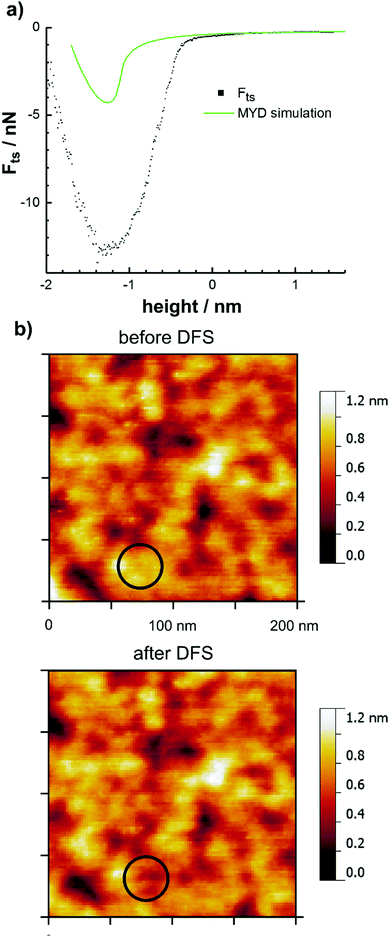
The measured frequency shift Δf curves were deconvoluted to the related tip–sample forces Fts using the method of Sader and Jarvis and a typical force curve is shown in Fig. 3a.13 In order to investigate the effect of the softness of the polymeric substrate, a simulated approach curve based on the MYD model was included for comparison.39 This model describes the contact mechanics based on elastic interactions due to van der Waals forces continuously not only in the contact, but also in the near-contact region. As discussed below, the required non-retarded Hamaker constant for the interface between polycarbonate and the surface cleansed TiAlN was not available but was approximated by the constant of the mixed Ti0.21Al0.45O surface oxide in contact with polycarbonate.
The simulated curve shows at a height of around −1 nm a rapid contact formation during the approach due to the formation of an instable neck as a result of the softness of the polycarbonate.38 However, when comparing the simulated and experimental curves, it follows that these elastic interactions do not describe the situation with sufficient accuracy. As discussed in the following, the shape of the force curve is rather explained by inelastic deformation and the reversible formation of single polymer bridges.
The occurrence of inelastic deformation was revealed by measuring the surface topography before and after DFS as shown in Fig. 3b. The images clearly show that the surface within the contact area deformed during the measurement and this was observed for all investigated tips.
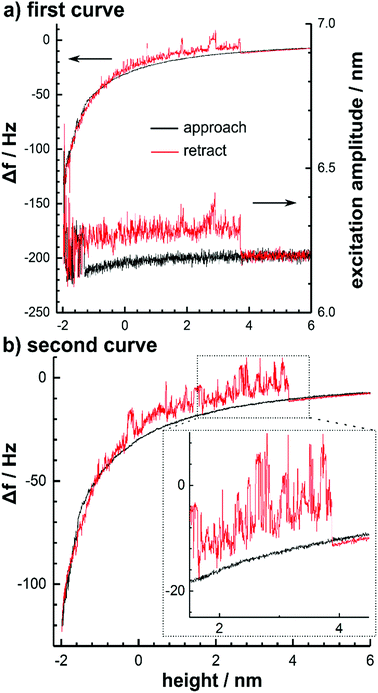
The reversible formation of molecular bridges was observed at a low oscillation amplitude of 3.3 nm in the case of the surface cleansed TiAlN and TiAl(O,N) tips due to strong interaction forces. Fig. 4 shows two typical consecutively measured frequency shift curves.
Upon approach, a positive frequency shift and an increased noise accompanied by an increased excitation amplitude was observed to set in within the non-contact region around −70 Hz (corresponding to 0.3 nN). This behaviour was preserved during retraction and the frequency shift was characterized by discontinuities until a height of around 4 nm. Above this height, the initial behaviour was rapidly recovered. When considering that this behaviour was not observed for large amplitudes, this points to the formation of molecular bridges between the tip and the substrate with a length of around 7 nm. The molecular bridges are thus only stable during an oscillation cycle at lower amplitudes. This interpretation is further corroborated by the fact that the frequency shift induced by the bridge is positive.
This was also observed recently when stretching a dextrane molecule linked to a vibrating cantilever tip and is a result of the increase of the restoring force with separation.9 Consequently, the question arises how many polymer chains contribute to bridge formation. As shown in Fig. 4, the stripping-off occurs repeatedly at a height of 4 nm and it is therefore reasonable to assume that basically only a single macromolecule forms the bridge. This is also consistent with the small interaction area involved. The energy dissipation caused by the presence of the molecular polymer bridge tethering the cantilever to the surface was calculated from the excitation amplitude and amounted around 320 eV per oscillation cycle. Since discontinuities were observed in the frequency shift, which require the presence of non-conservative hysteretic forces, the dissipation is explained by inelastic processes as a result of possibly viscoelastic effects or molecular processes induced by the polymer bridge entangled in the surface. In this regards, the related inelastic forces do not significantly contribute to the total interaction force and to adhesion. This follows from the fact that the frequency shift curve of the tethered cantilever is still highly similar to the unbound situation; the differences in the frequency shift are at maximum 20 Hz and would contribute to the complete frequency shift curve as shown in Fig. 2a only with 1.4 Hz after rescaling to the corresponding amplitude.12
Adhesion forces: structural and electronic origins
The interactions between the TiAlN coated tips and the polymer films were investigated after surface cleansing and after O beam oxidation of the sputter coating. From a chemical perspective no chemical bonds are expected to form between polystyrene and the surface cleansed TiAlN due to its termination with a surface oxide/oxynitride. Consequently, the interactions between the surface cleansed TiAlN and polystyrene are assumed to be purely governed by van der Waals interactions.The results are shown in Table 3. It was observed that the adhesion forces of the surface cleansed TiAlN tip on polycarbonate and polystyrene were not significantly different. This indicates that the carbonate moiety does not significantly affect the adhesion forces and that the interactions between the polycarbonate and the surface cleansed TiAlN coating are governed by van der Waals forces, too.
| Tip | Surface chemical structure | Polymer | F ad (nN) | Bridge formation |
|---|---|---|---|---|
| Surface cleansed TiAlN | TiAlN|TiAl(O,N)|(Ti(IV),Al) oxide | PC | 13.5 ± 1.1 | Yes |
| Surface cleansed TiAlN | TiAlN|TiAl(O,N)|(Ti(IV),Al) oxide | PS | 14.3 ± 1.3 | Yes |
| O beam oxidized TiAlN | TiAlN|TiAl(O,N)|Ti0.21Al0.45O | PC | 6.9 ± 0.70 | No |
In contrast, the adhesion force was decreased from 13.5 to 6.9 nN and no molecular bridges were observed after performing the O beam oxidation of the hard coating. This can be explained by a decrease of the van der Waals forces due to the formation of the 2.0 nm thick Ti0.21Al0.45O oxide layer which is accompanied by a loss of polarizability, because the free d-electrons are captured by oxygen during oxidation.
More interestingly, this shows that in the case of the surface cleansed TiAlN the adhesion is substantially increased by the oxynitride layer and possibly also the pure nitride phase beneath. This interpretation is consistent with a recent electrochemical characterization of the TiAlN hard coating where the oxynitride layer was shown to be highly polarizable.35
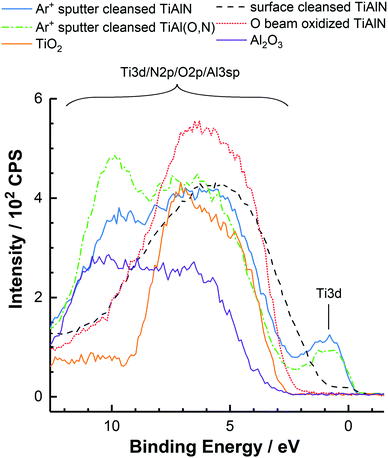
Since the van der Waals forces are ultimately related to the electronic properties of the materials, the samples were investigated by XPS valence band spectroscopy. Fig. 5 shows the spectra of TiAlN as obtained after surface cleansing and O beam oxidation. For comparison, the spectra of TiAlN and also that of the TiAl(O,N) coating are included as obtained after removal of the surface oxides by means of Ar+ sputter cleansing.
The valence band spectra of all samples were characterized by two bands located between 11 to 3 eV and at 0.5 eV, which are ascribed to the mixed N 2p/O 2p/Al 3p band hybridized with Ti 3d states and to the Ti 3d band.40 As shown earlier by REELS, these bands provide the optical response of the surfaces up to energies of around 15 eV.40 In contrast, higher binding energy levels present in the valence band such as the N 2s and O 2s levels are not relevant for the optical properties. Thus, only the p- and d-band related transitions can be considered to determine the dispersive properties of the surfaces.
When comparing all valence band spectra it was found that the d-electron density and therefore the amount of metal-like electrons were highest in the Ar+ sputter cleansed TiAlN and TiAl(O,N) coatings. In the case of the surface cleansed TiAlN the d-electron density was decreased as a result of oxidation at ambient conditions after the deposition of the hard coating. In addition to that, also the band gap region between the p- and d-bands at around 1.5 eV was filled with states from the p-band probably as a result of nitrogen doping.7 Following O beam oxidation the occupied states near the Fermi level up to a binding energy of 2.5 eV were basically completely removed. These findings clearly show that significant amounts of states close to the Fermi level are occupied in the near-surface region of the surface cleansed TiAlN in contrast to the O beam oxidized case. This in turn indicates an increased polarizability of the surface cleansed TiAlN in agreement with the measured adhesion forces.
Moreover, the valence band spectra disclosed a high degree of resemblance of the valence band structures of Ar+ sputter cleansed TiAlN and TiAl(O,N). Only the d-electron density in the Ar+ sputter cleansed TiAl(O,N) was decreased by around 13% and the intensity around 10 eV was increased as a result of increased O 2p contributions.41 This shows that the exchange of lattice nitrogen atoms in TiAlN with oxygen to form TiAl(O,N) does not require the loss of the metallic properties in terms of the Ti 3d electron density. Thus, the characterization of the adhesive properties of the TiAlN hard coating so far should also principally apply to the case of TiAl(O,N) coatings. This supposition was validated by measuring the adhesion on polycarbonate with a TiAl(O,N) coated tip after oxidation due to exposure to atmosphere and surface cleansing. As shown in Table 4 the adhesion was 0.53 ± 0.10 nN nm−1 and did thus not significantly differ from the adhesion measured with the surface cleansed TiAlN tip, which was around 0.60 ± 0.12 nN nm−1.
| Interface | A h (10−21 J) | F ad (nN nm−1) | Polymer bridging |
|---|---|---|---|
| a As measured with the O beam oxidized TiAlN tip covered with 2 nm Ti0.21Al0.45O. | |||
| Surface cleansed TiAlN|PC | — | 0.60 ± 0.12 | Yes |
| Surface cleansed TiAl(O,N)|PC | — | 0.53 ± 0.10 | Yes |
| Ti0.21Al0.45O|PC | 134 | 0.29 ± 0.06a | No |
| TiO2|PC | 149 | 0.52 ± 0.10 | No |
| Al2O3|PC | 111 | 0.31 ± 0.06 | No |
So far, the influence of the only partially oxidized and highly polarizable sublayers of surface cleansed TiAlN on the adhesion forces has been investigated. In the following, the role of the Ti(IV) and Al(III) ions in the Ti0.21Al0.45O mixed oxide, which terminates the surface of TiAlN after the intense O beam oxidation, is clarified based on DFS measurements and a complementary Lifshitz calculation of non-retarded Hamaker constants.
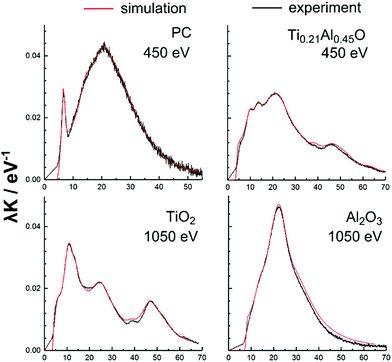
The calculation was based on optical data derived from REELS spectra measured on smooth amorphous TiO2 and Al2O3 PEALD films samples after removal of organic contaminations by surface cleansing. The advantage of the determination of the optical data by means of REELS is that the Ti0.21Al0.45O mixed oxide can be investigated directly in form of the 2 nm thin surface oxide layer on the O beam oxidized TiAlN sample. This is possible due to the low energy of the primary electron beam (450 eV) resulting into a depth-information of around 1 nm. The same beam energy was also used for the polycarbonate and polystyrene surfaces in order to avoid beam damage.23 The beam energy used for the TiO2 and Al2O3 samples was instead set to 1050 eV corresponding to a depth-information of around 2 nm in order to mitigate possible surface effects. The optical data of these materials were determined in form of the band gaps and the UV dielectric functions by quantitative evaluation of the REELS spectra using the QUEELS-ε(ω,k)-REELS software package.23,42,43 It is noted that this procedure involves first the separation of the main elastic peak in a REELS spectrum from the loss region, which is then deconvoluted in order to correct for losses from multiple scattering events and to obtain then the experimental single electron scattering cross section λK. This cross section is on the other hand simulated based on an empirical dielectric function, which is optimized until optimal agreement between experiment and simulation is found. However, in the case of the TiAlN and TiAl(O,N) hard coatings intraband transitions remained unresolved and these materials could thus not be analysed due to an erroneous experimental cross section.
The experimental and simulated inelastic scattering cross sections λK of polycarbonate, TiO2, Al2O3 and the Ti0.21Al0.45O surface oxide are shown in Fig. 6 for comparison. An excellent agreement was found between the experimental and theoretical curves illustrating the high quality of the dielectric function. In this regards, the band gaps used for the dielectric functions were 4.2 eV for polycarbonate and 3.2 eV for TiO2 and thus in agreement with literature.44 The band gap of Al2O3 was found to be 6.2 eV and was thus decreased when compared with the literature values of γ-Al2O3 (7.1 eV) and α-Al2O3 (8.4 eV) as a result of its amorphous structure.45 The band gap of the mixed Ti0.21Al0.45O could be determined to 3.4 eV. This value is close to TiO2 and reveals that the optical response of Ti0.21Al0.45O near the band gap originates from Ti 3d-band related transitions due to the presence of the Ti(IV) ion.
The influence of this electronic effect in Ti0.21Al0.45O on adhesion is investigated in the following by calculating the non-retarded Hamaker constants across vacuum based on the UV dielectric functions. It is noted that generally the consideration of UV frequencies above the cut-off wavelength only as in the present case is supposed to be sufficient for typical insulators.46 The calculation of the non-retarded constants was based on the exact Lifshitz formula for non-magnetic materials and is along with a parametric description of the dielectric functions documented in the ESI.† The non-retarded Hamaker constants of the different tip–sample contacts are shown in Table 4. The non-retarded Hamaker constants were 149 × 10−21 J for TiO2|PC, 134 × 10−21 J for the mixed Ti0.21Al0.45O|PC and 111 × 10−21 J for Al2O3|PC. Consequently, the dispersive interactions of Ti0.21Al0.45O with polycarbonate increase with the Ti/Al ratio as a result of an enhanced polarizability due to contributions of d-band related optical transitions caused by the Ti(IV) ion. Further, the Hamaker constant of Ti0.21Al0.45O is located in between that of the pure Ti- and Al-oxides and therefore follows the bulk Ti/Al metal ratio of the TiAlN, which is around 1.
In comparison, the corresponding experimental adhesion forces of TiO2 and Al2O3 were 0.52 and 0.31 nN nm−1 and that of the Ti0.21Al0.45O covered tip 0.29 nN nm−1. Considering that the accuracy is not better than 20% when comparing different cantilevers as a result of the spring and sensitivity calibrations and that also the slightly different tip shapes affect the comparability (see the ESI,† for electron microscopy images of the tips employed), these findings are generally in good agreement with the calculated Hamaker constants.
Moreover, the formation of polymer bridges between polycarbonate and TiO2 and Al2O3 was not observed (as opposed to the case of TiAlN and TiAl(O,N)), which is in agreement with the observed lower adhesion forces. The valence band spectra of TiO2 and Al2O3 shown in Fig. 5 show a structureless band gap without the presence of significant amounts of occupied states below the Fermi level, which reflect the insulating character of TiO2 and Al2O3 and therefore their decreased polarizability compared with TiAlN or TiAl(O,N).
Conclusions
The surface chemical analysis of the TiAlN hard coating by XPS showed that the surface was oxidized due to exposure to atmosphere after deposition and could be described by a complex multi-layered surface oxide consisting of an oxynitridic growth region covered by a fully oxidized top layer including Ti(IV) ions. It was further shown that organic contaminations present on this surface could be effectively removed by an in vacuo surface cleansing procedure involving an oxygen beam without significantly oxidizing the hard coatings surface itself (“surface cleansed”). This surface treatment enabled defined adhesion DFS measurements by means of the UHV-AFM.The investigation of the interaction forces between spin-cast PC and a cantilever coated with surface cleansed TiAlN by means of DFS was shown to be reversible without the occurrence of material transfer. However, single-molecular polymer bridges formed reversibly between the tip and the polycarbonate as a result of the strong interactions between these materials. In this regards, the analysis of the adhesion force showed that the interactions were governed by van der Waals forces, which mainly originate from the interactions of the polycarbonate with the only partially oxidized and therefore highly polarizable subsurface region of the coating. In agreement with this result, intense surface oxidation of the TiAlN coated tip decreased the adhesion force by a factor of two due to the formation of a 2.0 nm thick mixed Ti0.21Al0.45O oxide top layer and inhibited the formation of molecular bridges.
The electronic origin of the high polarizability of the subsurface region of surface cleansed TiAlN was revealed by XPS valence band spectroscopy and could be related to a joint effect of residual metal-like Ti 3d electrons at the Fermi level and the presence of p-band related occupied states at low binding energies. Further, the comparison of the valence band spectra of TiAlN and TiAl(O,N) after removal of the surface oxides showed that the formation of TiAl(O,N) by exchange of lattice nitrogen atoms in TiAlN with oxygen does basically not affect the metallic properties of the coating. As supported by adhesion force measurements, the dispersive interaction forces of TiAl(O,N) hard coatings can thus also be understood based on the TiAlN model system employed in this study.
In the case of fully oxidized TiAlN, which is covered by Ti0.21Al0.45O, the combined analysis of REELS spectra, non-retarded Hamaker constants and adhesion forces revealed that the Ti(IV) ion present in the mixed oxide provides Ti 3d band related transitions close to the band gap of 3.4 eV and thereby enhances the polarizability of the oxide. In consequence, also the van der Waals and adhesion forces increase with the Ti/Al ratio of the mixed oxide.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We gratefully acknowledge the financial supports of the German Research Foundation within the collaboration research centre SFB TR-87 subprojects A2, A3 and B4. We also kindly thank Holger Rueß and Prof. Dr Jochen M. Schneider from the Materials Chemistry division of the RWTH Aachen for providing the hard coatings.References
- E. J. Bienk and N. J. Mikkelsen, Application of advanced surface treatment technologies in the modern plastics moulding industry, Wear, 1997, 207, 6–9 CrossRef CAS.
- K. Bobzin, R. Nickel, N. Bagcivan and F. D. Manz, PVD—Coatings in Injection Molding Machines for Processing Optical Polymers, Plasma Processes Polym., 2007, 4, S144–S149 CrossRef.
- C. Gnoth, C. Kunze, M. Hans, M. Baben, J. Emmerlich, J. M. Schneider and G. Grundmeier, Surface chemistry of TiAlN and TiAlNO coatings deposited by means of high power pulsed magnetron sputtering, J. Phys. D: Appl. Phys., 2013, 46, 084003 CrossRef.
- S. Hofmann and H. A. Jehn, Selective oxidation and chemical state of Al and Ti in (Ti,Al)n coatings, Surf. Interface Anal., 1988, 12, 329–333 CrossRef.
- S. Hofmann, Formation and diffusion properties of oxide films on metals and on nitride coatings studied with Auger electron spectroscopy and X-ray photoelectron spectroscopy, Thin Solid Films, 1990, 193–194(Part 2), 648–664 CrossRef CAS.
- C. Kunze, D. Music, M. Baben, J. M. Schneider and G. Grundmeier, Temporal evolution of oxygen chemisorption on TiAlN, Appl. Surf. Sci., 2014, 290, 504–508 CrossRef CAS.
- M. Wiesing, T. de los Arcos and G. Grundmeier, The Thermal Oxidation of TiAlN High Power Pulsed Magnetron Sputtering Hard Coatings as Revealed by Combined Ion and Electron Spectroscopy, Adv. Mater. Interfaces, 2017, 1600861 CrossRef.
- P. U. D. Schwarz and D. H. Hölscher, in Scanning Probe Microscopy, ed. S. Kalinin and A. Gruverman, Springer, New York, 2007, pp. 506–533 Search PubMed.
- H. Hölscher, D. Ebeling, J.-E. Schmutz, M. M. Schäefer and B. Anczykowski, in Scanning Probe Microscopy in Nanoscience and Nanotechnology, ed. B. Bhushan, Springer Berlin Heidelberg, 2010, pp. 3–21 Search PubMed.
- M. Z. Baykara and U. D. Schwarz, in Noncontact Atomic Force Microscopy, ed. S. Morita, F. J. Giessibl, E. Meyer and R. Wiesendanger, Springer International Publishing, 2015, pp. 9–28 Search PubMed.
- A. Schirmeisen, H. Hölscher and U. D. Schwarz, in Noncontact Atomic Force Microscopy, ed. S. Morita, F. J. Giessibl and R. Wiesendanger, Springer Berlin Heidelberg, 2009, pp. 95–119 Search PubMed.
- F. J. Giessibl, Forces and frequency shifts in atomic-resolution dynamic-force microscopy, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 16010–16015 CrossRef CAS.
- J. E. Sader and S. P. Jarvis, Accurate formulas for interaction force and energy in frequency modulation force spectroscopy, Appl. Phys. Lett., 2004, 84, 1801–1803 CrossRef CAS.
- M. Guggisberg, M. Bammerlin, C. Loppacher, O. Pfeiffer, A. Abdurixit, V. Barwich, R. Bennewitz, A. Baratoff, E. Meyer and H.-J. Güntherodt, Separation of interactions by noncontact force microscopy, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 11151–11155 CrossRef CAS.
- E. T. Herruzo, H. Asakawa, T. Fukuma and R. Garcia, Three-dimensional quantitative force maps in liquid with 10 piconewton, angstrom and sub-minute resolutions, Nanoscale, 2013, 5, 2678–2685 RSC.
- A. Schirmeisen, D. Weiner and H. Fuchs, Measurements of metal–polymer adhesion properties with dynamic force spectroscopy, Surf. Sci., 2003, 545, 155–162 CrossRef CAS.
- O. Hunold, M. Wiesing, T. de los Arcos, D. Music, G. Grundmeier and J. M. Schneider, Influence of O2 exposure on the interaction between CH4 and amorphous AlYB14, Appl. Surf. Sci., 2017, 392, 1165–1172 CrossRef CAS.
- D. Music, D. Lange, L. Raumann, M. Baben, F. von Fragstein and J. M. Schneider, Polypropylene–MAlN (M = Ti, Cr) interface interactions, Surf. Sci., 2012, 606, 986–989 CrossRef CAS.
- R. T. Haasch, T.-Y. Lee, D. Gall, J. E. Greene and I. Petrov, Epitaxial TiN(001) Grown and Analyzed In situ by XPS and UPS. II. Analysis of Ar+ Sputter Etched Layers, Surf. Sci. Spectra, 2000, 7, 204–212 CrossRef CAS.
- D. C. Bradley and I. M. Thomas, 765. Metallo-organic compounds containing metal–nitrogen bonds. Part I. Some dialkylamino-derivatives of titanium and zirconium, J. Chem. Soc., 1960, 3857–3861 RSC.
- M. Gebhard, F. Mitschker, M. Wiesing, I. Giner, B. Torun, T. de los Arcos, P. Awakowicz, G. Grundmeier and A. Devi, An efficient PE-ALD process for TiO2 thin films employing a new Ti-precursor, J. Mater. Chem. C, 2016, 4, 1057–1065 RSC.
- C. J. Powell and A. Jablonski, NIST Electron Effective-Attenuation-Length Database, National Institute of Standards and Technology, Gaithersburg, MD, 2011 Search PubMed.
- D. Tahir and S. Tougaard, Electronic and optical properties of selected polymers studied by reflection electron energy loss spectroscopy, J. Appl. Phys., 2012, 111, 054101 CrossRef.
- C. Corbella, S. Grosse-Kreul, O. Kreiter, T. de los Arcos, J. Benedikt and A. von Keudell, Particle beam experiments for the analysis of reactive sputtering processes in metals and polymer surfaces, Rev. Sci. Instrum., 2013, 84, 103303 CrossRef PubMed.
- R. Anton, T. Wiegner, W. Naumann, M. Liebmann, C. Klein and C. Bradley, Design and performance of a versatile, cost-effective microwave electron cyclotron resonance plasma source for surface and thin film processing, Rev. Sci. Instrum., 2000, 71, 1177–1180 CrossRef CAS.
- J. E. Sader, J. A. Sanelli, B. D. Adamson, J. P. Monty, X. Wei, S. A. Crawford, J. R. Friend, I. Marusic, P. Mulvaney and E. J. Bieske, Spring constant calibration of atomic force microscope cantilevers of arbitrary shape, Rev. Sci. Instrum., 2012, 83, 103705 CrossRef PubMed.
- M. J. Higgins, R. Proksch, J. E. Sader, M. Polcik, S. M. Endoo, J. P. Cleveland and S. P. Jarvis, Noninvasive determination of optical lever sensitivity in atomic force microscopy, Rev. Sci. Instrum., 2006, 77, 013701 CrossRef.
- G. H. Simon, M. Heyde and H.-P. Rust, Recipes for cantilever parameter determination in dynamic force spectroscopy: spring constant and amplitude, Nanotechnology, 2007, 18, 255503 CrossRef.
- S. Hofmann, Auger- and X-Ray Photoelectron Spectroscopy in Materials Science, Springer, 2013 Search PubMed.
- S. Hofmann, Sputter depth profile analysis of interfaces, Rep. Prog. Phys., 1998, 61, 827 CrossRef CAS.
- L. Porte, L. Roux and J. Hanus, Vacancy effects in the X-ray photoelectron spectra of TiNx, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 28, 3214–3224 CrossRef CAS.
- A. Arranz and C. Palacio, Screening effects in the Ti 2p core level spectra of Ti-based ternary nitrides, Surf. Sci., 2006, 600, 2510–2517 CrossRef CAS.
- G. Greczynski and L. Hultman, Self-consistent modelling of X-ray photoelectron spectra from air-exposed polycrystalline TiN thin films, Appl. Surf. Sci., 2016, 387, 294–300 CrossRef CAS.
- P. Prieto and R. E. Kirby, X-ray photoelectron spectroscopy study of the difference between reactively evaporated and direct sputter-deposited TiN films and their oxidation properties, J. Vac. Sci. Technol., A, 1995, 13, 2819–2826 CAS.
- M. Wiesing, M. Baben, J. M. Schneider, T. de los Arcos and G. Grundmeier, Combined Electrochemical and Electron Spectroscopic Investigations of the Surface Oxidation of TiAlN HPPMS Hard Coatings, Electrochim. Acta, 2016, 208, 120–128 CrossRef CAS.
- R. Cremer, M. Witthaut and D. Neuschütz, Electron spectroscopy applied to metastable ceramic solution phases, Fresenius’ J. Anal. Chem., 1999, 365, 28–37 CrossRef CAS.
- B. Gotsmann and H. Fuchs, The measurement of hysteretic forces by dynamic AFM, Appl. Phys. A: Mater. Sci. Process., 2014, 72, S55–S58 CrossRef.
- D. Krüger, B. Anczykowski and H. Fuchs, Physical properties of dynamic force microscopies in contact and noncontact operation, Ann. Phys., 1997, 509, 341–363 CrossRef.
- V. M. Muller, V. S. Yushchenko and B. V. Derjaguin, General theoretical consideration of the influence of surface forces on contact deformations and the reciprocal adhesion of elastic spherical particles, J. Colloid Interface Sci., 1983, 92, 92–101 CrossRef CAS.
- I. leR. Strydom and S. Hofmann, XPS and EELS study of the valence band electronic structure of TiN and (Ti,Al)N coatings as influenced by the deposition parameters, Vacuum, 1990, 41, 1619–1623 CrossRef CAS.
- M. Baben, L. Raumann and J. M. Schneider, Phase stability and elastic properties of titanium aluminum oxynitride studied by ab initio calculations, J. Phys. D: Appl. Phys., 2013, 46, 084002 CrossRef.
- S. Tougaard and F. Yubero, QUEELS software package for calculation of surface effects in electron spectra, Surf. Interface Anal., 2004, 36, 824–827 CrossRef CAS.
- S. Tougaard and I. Chorkendorff, Differential inelastic electron scattering cross sections from experimental reflection electron-energy-loss spectra: Application to background removal in electron spectroscopy, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 35, 6570–6577 CrossRef CAS.
- M. D. Migahed and H. M. Zidan, Influence of UV-irradiation on the structure and optical properties of polycarbonate films, Curr. Appl. Phys., 2006, 6, 91–96 CrossRef.
- D. Tahir, H. L. Kwon, H. C. Shin, S. K. Oh, H. J. Kang, S. Heo, J. G. Chung, J. C. Lee and S. Tougaard, Electronic and optical properties of Al2O3/SiO2 thin films grown on Si substrate, J. Phys. D: Appl. Phys., 2010, 43, 255301 CrossRef.
- V. A. Parsegian, van der Waals Forces: A Handbook for Biologists, Chemists, Engineers, and Physicists, Cambridge University Press, Cambridge, 2005 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Complementary spectroscopic data, parameters and calculations. See DOI: 10.1039/c7cp05373h |
| This journal is © the Owner Societies 2018 |