Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface induced smectic order in ionic liquids – an X-ray reflectivity study of [C22C1im]+[NTf2]−†
Julian
Mars
 ab,
Binyang
Hou‡
ab,
Binyang
Hou‡
 ac,
Henning
Weiss
a,
Hailong
Li
a,
Oleg
Konovalov
c,
Sven
Festersen
d,
Bridget M.
Murphy
ac,
Henning
Weiss
a,
Hailong
Li
a,
Oleg
Konovalov
c,
Sven
Festersen
d,
Bridget M.
Murphy
 de,
Uta
Rütt
f,
Markus
Bier
de,
Uta
Rütt
f,
Markus
Bier
 gh and
Markus
Mezger
gh and
Markus
Mezger
 *ab
*ab
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: mezger@mpip-mainz.mpg.de
bInstitute of Physics and MAINZ Graduate School, Johannes Gutenberg University Mainz, 55128 Mainz, Germany
cESRF The European Synchrotron, 71 avenue des Martyrs, 38000 Grenoble, France
dInstitute of Experimental and Applied Physics, Kiel University, Leibnizstr. 19, 24098 Kiel, Germany
eRuprecht Haensel Laboratory, Kiel University, Leibnizstr. 19, 24098 Kiel, Germany
fDESY Photon Science, Notkestr. 85, 22607 Hamburg, Germany
gMax Planck Institute for Intelligent Systems, Heisenbergstr. 3, 70569 Stuttgart, Germany
hInstitute for Theoretical Physics IV, University of Stuttgart, Pfaffenwaldring 57, 70569 Stuttgart, Germany
First published on 26th September 2017
Abstract
Surface induced smectic order was found for the ionic liquid 1-methyl-3-docosylimidazolium bis(trifluoromethlysulfonyl)imide by X-ray reflectivity and grazing incidence scattering experiments. Near the free liquid surface, an ordered structure of alternating layers composed of polar and non-polar moieties is observed. This leads to an oscillatory interfacial profile perpendicular to the liquid surface with a periodicity of 3.7 nm. Small angle X-ray scattering and polarized light microscopy measurements suggest that the observed surface structure is related to fluctuations into a metastable liquid crystalline SmA2 phase that was found by supercooling the bulk liquid. The observed surface ordering persists up to 157 °C, i.e. more than 88 K above the bulk melting temperature of 68.1 °C. Close to the bulk melting point, we find a thickness of the ordered layer of L = 30 nm. The dependency of L(τ) = Λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) ln(τ/τ1) vs. reduced temperature τ follows a logarithmic growth law. In agreement with theory, the pre-factor Λ is governed by the correlation length of the isotropic bulk phase.
ln(τ/τ1) vs. reduced temperature τ follows a logarithmic growth law. In agreement with theory, the pre-factor Λ is governed by the correlation length of the isotropic bulk phase.
1 Introduction
Ionic Liquids (ILs) are organic salts with a melting point below 100 °C. They are typically composed of bulky and asymmetric cations, containing a positively charged moiety, polar groups and/or aliphatic side chains. The balance between Coulomb, polar, van der Waals interactions and hydrogen bonding, leads to complex structures in the liquid phase. This results in remarkable solvent properties, that are rarely found in other materials and can be tailored by the selection of a specific anion and cation combination.1,2 Over the last three decades, synthesis of new ILs that are stable under ambient conditions opened up new fields for applications.3 Thus, ionic liquids hold immense promise as environmentally friendly replacements for conventional solvents and reaction media in chemical, energy and nano applications.2,4 Most of these applications involve processes at surfaces and interfaces in contact with other media. In the SILP (Supported Ionic Liquids Heterogeneous Phase Catalysis) process the chemical reaction takes place in an IL thin film, wetting a solid support material with high surface area.5–7 Here, one rate limiting step is the diffusion of reactants and products across the IL/gas interface. Therefore, to better understand the interfacial transport mechanisms and optimize the performance of ILs in SILP catalysis a detailed knowledge of the IL surface structure is highly desirable.8The bulk structure of ILs was extensively studied experimentally by X-ray scattering,9–19 neutron scattering10,11,13,20 coherent anti-Stokes Raman scattering21 and by computer simulations.16,22–29 One particular feature, that draws significant attention, is the appearance of a so-called pre-peak in scattering experiments.16 These pre-peaks were observed for a wide range of ILs with aliphatic side chains longer than a butyl group. Corresponding to a length scale of a few nanometers, this peak was attributed to a high degree of intermediate range mesoscopic order. It is caused by micro phase separation in the polar networks composed of the charged moieties and domains of the non-polar alkyl side chains.24 Beyond this intermediate range mesoscopic order, long range translational order is observed in Ionic Liquid Crystals (ILCs).9,30–33 Such ILCs with long aliphatic side chains can form smectic mesophases over an extended temperature range.34,35 From computer simulations, a detailed understanding of their phase behavior depending on electrostatic and vdW interactions has been obtained.36–40
These bulk heterogeneities affect their molecular scale arrangement at surfaces and interfaces. Simulations of imidazolium based IL surfaces indicate that the aliphatic part of the cation points towards the interface. Nevertheless, due to the low packing of the aliphatic alkyl-chains, the aromatic ring as well as the alkyl-chains were found to be present within the uppermost surface region.41–43 Experimentally, angle-resolved X-ray photoelectron spectroscopy shows an enrichment of the aliphatic chains at the interface of [CnC1im]+-based ILs with n > 2.44–49 Smaller anions tend to give a stronger enrichment. These observations are consistent with sum frequency generation spectroscopy showing that the imidazolium ring is parallel to the surface and the aliphatic chain is pointing toward the gas phase.50,51 Interfacial profiles with sub-nanometer resolution across free IL surfaces52–55 and buried interfaces56,57 were obtained by X-ray reflectivity (XRR) experiments.58,59 Jeon et al. observed a surface crystalline layer in [C4C1im]+[PF6]− using grazing incident X-ray diffraction (GIXD).60 Using resonant soft X-ray reflectivity, it was shown that the micro phase separation in ILs with long aliphatic side-chains drives an oscillatory near surface structure with a periodicity on the nanometer length scale.55 Towards the bulk, this structure is exponentially decaying with a correlation length matching the corresponding value found in the isotropic bulk liquid. Like in bulk, the oscillatory surface profiles originate from alternating regions enriched with ionic and aliphatic moieties.55 Recently, long ranged order has been found for ionic liquid films and in confinement.61,62
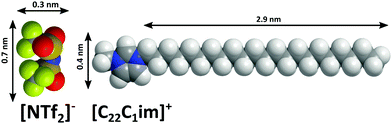
Here, we present a study of the 1-methyl-3-docosylimidazolium bis(trifluoromethlysulfonyl)imide ([C22C1im]+[NTf2]−, Fig. 1) surface. This imidazolium based IL with long C22 side chains exhibits pronounced microphase separation.63 Using X-ray reflectivity and grazing incidence scattering, we obtain information on the temperature dependent surface structure with molecular scale resolution. Complementary information from small angle X-ray scattering, polarized light microscopy and calorimetric measurements suggest that the observed surface structure is related to fluctuations into a metastable liquid crystalline phase that was found by supercooling the bulk liquid.
2 Materials and methods
2.1 Synthesis and characterization
[C22C1im]+[NTf2]− was synthesized from 1-methylimidazol (≥99%, Merck, Darmstadt), 1-bromodocosane (Santa Cruz Biotechnology, Dallas) and [Li]+[NTf2]− (>98%, Tokyo Chemical Industry, Tokyo). Subsequently, the IL was purified by recrystallization from a cold ethanol/water (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) mixture and zone melted in glass tubes under vacuum.64–66 Details on material synthesis and purification are described in ref. 67.
25) mixture and zone melted in glass tubes under vacuum.64–66 Details on material synthesis and purification are described in ref. 67.
The temperature dependent molecular volume V = Vm[1 + α(T − Tm)] of the bulk liquid was determined by pycnometry. Interpolation by linear regression yield a molecular volume of Vm = 1.015 nm3 at the melting point Tm = 68.1 °C and a volumetric thermal expansion coefficient α = 0.745 × 10−3 K−1.
Phase transition temperatures and entropies ΔS = ΔH/T of [C22C1im]+[NTf2]− were determined by differential scanning calorimetry (Mettler Toledo DSC-822) using scan rates between 1 K min−1 and 10 K min−1 (ESI†). Equilibrium phase transition temperatures were obtained by extrapolating to zero heating and cooling rates.
Polarized light microscopy (POM) was carried out using a 10× objective and wave plate between crossed polarizer (Zeiss Photomicroscope III). The IL was contained between two glass cover slips with a sample thickness of approx. 100 μm. Temperature dependent measurements at a rate of approx. 20 K min−1 were performed using a heating microscope stage (Linkam THMS600).
2.2 Bulk X-ray scattering
Bulk X-ray scattering data was measured in transmission geometry using Cu Kα, λ = 0.154 nm radiation (Rigaku MicroMax 007 X-ray generator, Osmic Confocal Max-Flux curved multilayer optics). The IL was contained in an 1 mm thick glass capillary (wall thickness 0.01 mm). Capillaries were placed in a nitrogen stream (Oxford Cryostream 700) and a temperature controlled copper holder inside a vacuum tube for wide and small angle scattering experiments respectively. Scattering data was recorded on an online image plate detector (Mar345, MarResearch) at a sample-detector distance of 352 mm and 2089 mm. Background from high energy radiation, was removed by a Laplace filter based masking algorithm. Scattering patterns I(q) vs. momentum transfer q = 4π/λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) sin(θ) were obtained by radial averaging of the 2D-scattering patterns.
sin(θ) were obtained by radial averaging of the 2D-scattering patterns.
2.3 X-ray reflectivity
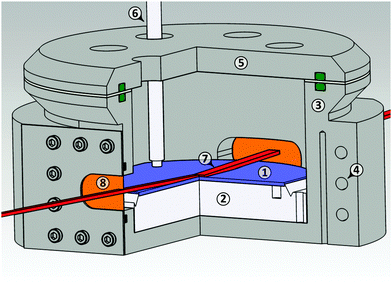
X-ray reflectivity (XRR) experiments were performed at the liquid interface scattering apparatus (LISA) at P08, PETRA III, DESY Hamburg.68 The size of the focussed X-ray beam (18 keV X-ray energy, λ = 0.069 nm) was 500 μm × 8 μm at the sample position. Scattering pattern was collected on a hybride pixel detector (Dectris Eiger 1M, 75 μm × 75 μm pixel size) at 1.2 m sample-detector distance. The liquid was contained in a circular PTFE sample trough (1 mm depth, 9 cm diameter). The trough was placed in a temperature controlled (Julabo FN25-HE) stainless steel (EN 1.4571) chamber (stability ±0.006 K) under helium atmosphere (Fig. 2). To improve the temperature homogeneity at the IL/helium interface, the steel cell was packed in 5 cm thick melamine isolation foam.The PTFE trough was cleaned in Piranha solution (3 parts H2SO4 98%, 1 part hydrogen peroxide 30%) for at least 20 minutes, thoroughly rinsed with ultrapure water, and dried in a nitrogen stream. [C22C1im]+[NTf2]− was filled into the heated trough at 90 °C until a convex meniscus was formed.
Molecular resolution is obtained by recording XRR patterns up to a sufficiently high momentum transfers qz perpendicular to the sample surface. Therefore, the specular reflected X-ray beam was recorded for incident angles αi ≤ 1.9°. This corresponds to momentum transfers qz = 4π/λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) αi ≤ 6 nm−1. Specular intensities were extracted from the 2D patterns by integration over an area of 40 × 40 detector pixels. The background signal predominantly originates from the diffuse scattering contributions of the bulk liquid. It exhibits an approximately concentric intensity distribution around the primary beam axis. Therefore, background intensities were measured at a horizontal offset of 20 pixels and a vertical offset, adjusted to keep the total scattering angle 2θ for the specular reflection and the background equal. XRR segments measured at different absorber settings were merged and interpolated on an equidistant qz-grid.
αi ≤ 6 nm−1. Specular intensities were extracted from the 2D patterns by integration over an area of 40 × 40 detector pixels. The background signal predominantly originates from the diffuse scattering contributions of the bulk liquid. It exhibits an approximately concentric intensity distribution around the primary beam axis. Therefore, background intensities were measured at a horizontal offset of 20 pixels and a vertical offset, adjusted to keep the total scattering angle 2θ for the specular reflection and the background equal. XRR segments measured at different absorber settings were merged and interpolated on an equidistant qz-grid.
2.4 Grazing incidence scattering
Grazing incident X-ray scattering experiments were performed at the six-circle liquid diffractometer at beamline ID10-EH1 at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France using an energy of 8 keV (λ = 0.155 nm). The IL was contained in a circular stainless steel trough (120 mm diameter, 1 mm depths). To reduce the background signal from air scattering the sample was kept under helium atmosphere during X-ray irradiation. First, the sample was heated to 96 °C followed by gradual cooling to the desired temperature with a rate of 0.25 K min−1. Signals were collected by a gas filled multi-channel detector (Gabriel 150 mm) in vertical orientation. Grazing incident X-ray scattering patterns were recorded at an incident angle αi = 0.12° i.e. 80% of the measured critical angle of total reflection αc = 0.15°.3 Analysis
3.1 X-Ray reflectivity
XRR can provide information on the density profile ρ(z) across liquid surfaces on a molecular length scale. For an ideal sharp surface profile, the so-called Fresnel reflectivity RF(qz) is obtained.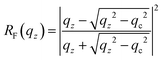 | (1a) |
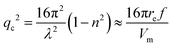 | (1b) |
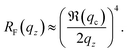 | (2) |
Deviations from an ideal surface cause modulations in the Fresnel normalized XRR patterns. Within the Born approximation, the XRR pattern R(qz) is linked to its corresponding real space electron density profile ρe(z) across the liquid surface via Fourier transformation72,73
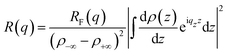 | (3) |
3.2 Model profiles and fitting procedure
Electron density profiles ρe(z) across IL surfaces were made up of different contributions: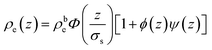 | (4) |
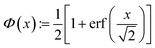 | (5) |
The oscillatory function with periodicity d is expanded in a series
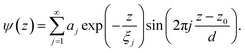 | (6) |
The oscillatory function ψ(z) in eqn (6) corresponds to the microscopic structure of smectic layers of the IL moieties. The envelope ϕ(z) in eqn (4) plays the role of a locally varying smectic order parameter profile. Within a simple variant of the Landau-de Gennes formalism,77 one can determine that smectic order parameter profile ϕ(z) of a smectic surface layer of thickness L in between a vapor phase and the bulk liquid. Here, only the leading terms
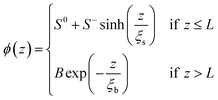 | (7) |
Reflectivity curves were numerically calculated using the Parratt formalism after dividing the profile into 0.05 nm slabs of constant density.72,78 Dispersion effects were included using X-ray form factors from Henke et al.69 Density profiles were extracted from the experimental XRR data by parameter refinement using a simulated annealing algorithm.79 Damping of the specular reflectivity due to capillary wave roughness of the liquid surface80,81 was taken into account by
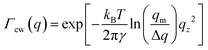 | (8) |
3.3 Surface thermodynamics
Within a generic approach, interface induced phase transitions such as wetting, interfacial premelting, or surface induced ordering can be described by a continuum model.83–85 In this model, a thin surface layer of thickness L is wetting the bulk. In the case of surface induced ordering, the bulk consists of an isotropic liquid (l). The surface layer (s), forming at the liquid/vapor (v) interface, exhibits a higher degree of order. This leads to a negative surface excess entropy.86 In the standard continuum model, the interfacial free energy of the system is expressed by an effective thickness-dependent γ(L).| γ(L) = γlv + φ(L)Δγ | (9a) |
| Δγ = γls + γsv − γlv | (9b) |
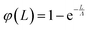 | (9c) |
By minimization of the total free energy of the system, a logarithmic growth law of the ordered surface layer thickness L vs. reduced temperature 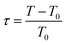 is obtained.
is obtained.
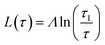 | (10a) |
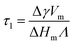 | (10b) |
4 Results and discussion
4.1 Bulk structure
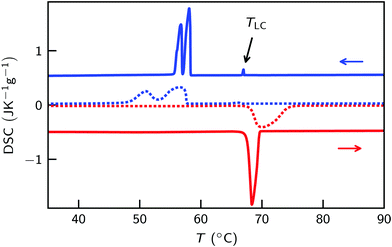
Upon heating, [C22C1im]+[NTf2]− shows a phase transition from the crystalline phase to an isotropic liquid at Tm = 68.1 (Fig. 3 and Fig. S1, ESI†). However, three phase transitions were observed by DSC during cooling. For the peak at TLC = 67.1 °C a transition entropy of ΔSLC = 2.5 J mol−1 K−1 was found (Fig. 3 and Fig. S2, ESI†). This resembles typical values for the formation of liquid crystalline mesophases.87 Similarly, a tendency to form liquid crystalline-like structures has been proposed for long aliphatic side chains by MS-CG simulations.36,37 The transition temperature is only about 1 K below the melting point. No corresponding transition was observed during heating. Therefore, we conclude that the transition is related to a metastable phase. For other imidazolium based ILs with long aliphatic C16 to C18 side chains and smaller [Cl]−, [BF4]− and [PF6]− anions thermodynamically stable SmA2-phases have been found.9,30–33Indeed, a focal conic texture, indicating a smectic A liquid crystalline mesophase, was observed in POM (Fig. 4c).63 Together with the SAXS data (Fig. 4) and the dimensions of the molecular moieties (Fig. 1) this mesophase is identified as a SmA2 liquid crystalline phase. While the absence of bâtonnets typically is linked to nematic to smectic phase transitions88 no signs for a nematic phase was found by DSC, POM and X-ray scattering. At 58.4 °C and 57.7 °C a double peak was observed in DSC. The higher temperature peak was attributed to the smectic to crystal transition. Its transition temperature strongly varied for different DSC measurements. The second peak is likely related to a solid–solid transition between two different crystal structures.
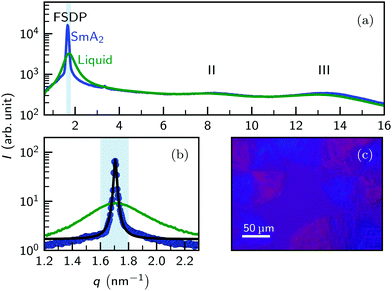
Above the melting point, the X-ray scattering patterns of [C22C1im]+[NTf2]− exhibit three diffuse peaks at q0 = 1.7 nm−1, 7.9 nm−1 and 12.9 nm−1 (Fig. 4a). The two peaks II and III at large momentum transfer q were associated with charge alternation and adjacency correlations.15,29 From the peak position, a periodicity 2π/q0 ≈ 3.6 nm is estimated at T = 96 °C. Having a periodicity similar to the molecular length scale (Fig. 1), the origin of the first sharp diffraction peak (FSDP) can be identified with the polarity alternation i.e. microphase separation of the ionic and aliphatic moieties.12,25,26,29
Quantitative analysis by fitting a generalized Teubner–Strey model to the scattering patterns yield a periodicity db(T) = 3.74 nm − 0.348 × 10−2 nm K−1 (T − Tm) and a correlation length ξb(T) = 4.79 nm − 0.0298 nm K−1 (T − Tm). A detailed X-ray scattering study of the [C22C1im]+[NTf2]− bulk structure in comparison to [C18C1im]+[FAP]−, [C18C1im]+[NTf2]−, [C18C1im]+[NNf2]− and [C22C1im]+[NNf2]− is found in ref. 67.
Upon rapid cooling below the SmA2 transition temperature TLC, the FSDP becomes much sharper. The periodicity dLC = 2π/q0 = 3.68 nm was found to be only 1.6% shorter then the value in the isotropic liquid. Albeit much weaker in intensity, a 2nd order reflection is observed at 2q0 = 3.4 nm−1. Analysis of high-resolution SAXS data by fitting a pseudo-Voigt function to the FSDP reveals a total FWHM of 0.0185 nm−1 (Fig. 4a). This value is close to the resolution limit of the instrument. From the Scherrer formula we estimate domain sizes ≥0.7 μm i.e. long ranged translational order. In contrast, the intensity, position and shape of the high-q peaks II and III remain almost unchanged. Together with the small change in the position of the FSDP, this indicates a strong structural similarity of the liquid crystalline phase and the mesoscopic structure found in the liquid phase.19
4.2 Surface structure
Density profile ρe(z) across the liquid surface with molecular scale resolution were extracted from the XRR patterns. Fig. 5a shows XRR curves R(qz) versus momentum transfer of the [C22C1im]+[NTf2]− surface for different temperatures above the bulk melting point. To highlight features originating from density modulations adjacent to the liquid surface, data was normalized to the Fresnel reflectivity RF(qz) of an structure-less surface (eqn (1)). For [C22C1im]+[NTf2]− we calculate a critical momentum transfer of total reflection ℜ(qc) = 0.23 nm−1.69 This corresponds to a critical incidence angle αc = 0.070° at 18 keV. From the experimental data we extract a critical momentum transfer qc = 0.21 nm−1. This agrees well with the value calculated from eqn (1b). As expected, below qc we obtain a reflectivity close to unity. Spikes in the region around qz ≈ qc are caused by the RF normalization with minute uncertainties in the calculated critical momentum transfer qc.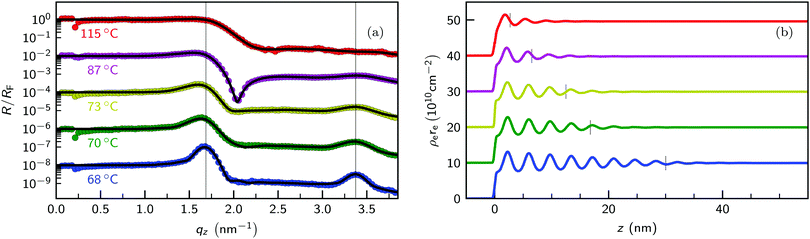 | ||
| Fig. 5 (a) Temperature dependent XRR curves of the [C22C1im]+[NTf2]− surface normalized to the Fresnel reflectivity RF(qz) (symbols) and calculated patterns (solid lines). Vertically dashed lines at 2π/ds = 1.69 nm−1 and 3.37 nm−1 indicate the positions of the 1st and 2nd order quasi-Bragg peaks. (b) Electron density profile ρe(z) obtained by fitting eqn (4) to the experimental XRR data. Horizontal tics indicate the layer thickness L of the ordered structure near the IL surface. Curves measured at 115 °C (red), 87 °C (purple), 73 °C (yellow), 70 °C (green), and 68 °C (blue) are vertically shifted for clarity. | ||
Deviations from an ideal surface cause modulations in the Fresnel normalized XRR patterns (Fig. 5). For the highest temperature of 115 °C (red symbols), a step-like reflectivity curve is obtained. At lower temperatures, this step gradually transforms into a dip (purple curve, 87 °C). These modulations are directly linked to changes in the density profiles across the IL surface by Fourier transformation (eqn (3)). Therefore, the observed dip, resembling an inverted Lorentzian peak, correspond to an exponentially damped oscillatory density profile across the IL surface.57,89 Qualitatively similar XRR curves were obtained for [C18C1im]+[FAP]−.55 Using resonant scattering techniques, the oscillatory surface profile was attributed to alternating layers. They are composed of aliphatic side chains and ionic moieties i.e. the anions and positively charged imidazolium rings. The periodicity ds = 2π/q0 ≈ 3 nm of the oscillatory surface profiles estimated from the peak position q0 = 2 nm−1 is similar to the bulk value db.
Below 73 °C (yellow symbols) two Bragg-like peaks emerge in the XRR curves. They correspond to 1st and 2nd order reflections at q0 = 1.68 nm−1 and 2q0 = 3.35 nm−1, respectively. As temperature decreases towards the transition temperature TLC where long ranged translational order was observed in bulk, these peaks get sharper and their intensities increase (green and blue symbols). Like the dip, these peaks originate from an oscillatory surface profile. However, the appearance of two distinct Bragg-like 1st and 2nd order peaks indicate an increasing surface order. In analogy, in the SAXS bulk measurements the 2nd order FSDP was only observed in the liquid crystalline smectic phase. This suggests the formation of surface induced smectic order with a smectic layer of thickness L wetting the isotropic liquid bulk phase.
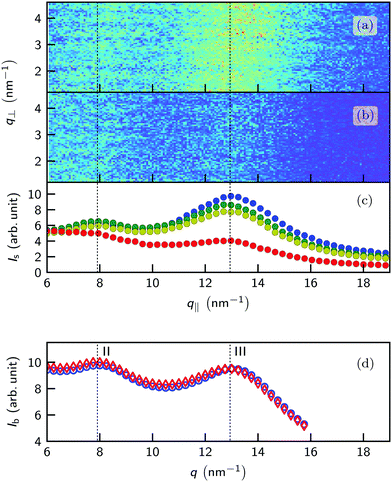
Fig. 6a shows the grazing incidence scattering patterns recorded from the [C22C1im]+[NTf2]− surface at 70.2 °C. At the grazing angle αi = 0.12°, corresponding to 80% of the critical angle of total reflection, the X-ray beam does not penetrate into the bulk liquid. Instead, an evanescent wave with a decay length of approx. 10 nm ≈ 3ds is formed. Therefore, in the grazing incidence experiments the bulk signal is strongly suppressed. This allows to detect the relatively weak scattering from the near surface region where oscillatory density profiles were observed. Long-range lateral order at the surface, e.g. a 2D crystalline structure, gives rise to sharp streaks along q⊥ at distinct q‖ values.90
However, in contrast to the experiments by Jeon et al. on [C4C1im]+[PF6]−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 at all temperatures no indications for long-ranged in-plane order have been found in our experiments. Instead, two broad diffuse peaks are observed (Fig. 6c). Their positions and widths are similar to the peaks II and III observed in bulk (Fig. 6d). However, unlike in bulk the relative peak intensity is strongly changing with temperature. Upon cooling, the intensity of peak III at 13 nm−1 is significantly increasing. In contrast, the corresponding bulk peak intensity remains almost unchanged.18 At low temperatures, the long axis of the smectic fluctuations adjacent to the surface are preferably aligned parallel to the surface normal. Thus, the in-plane scattering signals in the q‖-direction originating from charge alternation (peak II) and adjacency correlations (peak III) are enhanced. When the thickness of the smectic layer L reaches the decay length of the evanescent X-ray wave the peak intensities saturate. This texture effect affects only the peak intensities, leaving their position and shape unchanged with respect to the isotropic bulk liquid. This observation confirms our interpretation that the structure of the [C22C1im]+[NTf2]− surface above its melting point is governed by fluctuations of the metastable smectic mesophase rather than by formation of a 2D surface crystal.
60 at all temperatures no indications for long-ranged in-plane order have been found in our experiments. Instead, two broad diffuse peaks are observed (Fig. 6c). Their positions and widths are similar to the peaks II and III observed in bulk (Fig. 6d). However, unlike in bulk the relative peak intensity is strongly changing with temperature. Upon cooling, the intensity of peak III at 13 nm−1 is significantly increasing. In contrast, the corresponding bulk peak intensity remains almost unchanged.18 At low temperatures, the long axis of the smectic fluctuations adjacent to the surface are preferably aligned parallel to the surface normal. Thus, the in-plane scattering signals in the q‖-direction originating from charge alternation (peak II) and adjacency correlations (peak III) are enhanced. When the thickness of the smectic layer L reaches the decay length of the evanescent X-ray wave the peak intensities saturate. This texture effect affects only the peak intensities, leaving their position and shape unchanged with respect to the isotropic bulk liquid. This observation confirms our interpretation that the structure of the [C22C1im]+[NTf2]− surface above its melting point is governed by fluctuations of the metastable smectic mesophase rather than by formation of a 2D surface crystal.
4.3 Growth law
To obtain quantitative density profiles across the IL surface, the experimental XRR data was analyzed using eqn (4). For all temperatures, the experimental XRR pattern is perfectly reproduced by the curves calculated from the model profiles (Fig. 5). Model parameters of the best fits are summarized in Table 1. Detailed analysis showed that the values extracted for the smectic layer thickness L are robust parameters (ESI†).| T (°C) | L (nm) | ξ b (nm) | d (nm) | z 0 (nm) | σ s (nm) | S 0 | S − | B | a 2 | ξ 2 (nm) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Parameters were fixed to the interpolated bulk values extracted from SAXS.67 b Parameters were determined by continuity and differential continuity conditions. ξs was fixed at 2000 nm. | ||||||||||
| 68 | 30.0 ± 2.0 | 4.78 | 3.73 | 2.28 | 0.18 | 1.71 | −98 | 126 | 0.15 | 26 |
| 70 | 16.8 ± 2.2 | 4.73 | 3.73 | 2.29 | 0.17 | 1.64 | −152 | 12.6 | 0.15 | 18 |
| 73 | 12.5 ± 1.2 | 4.64 | 3.73 | 2.24 | 0.10 | 1.62 | −189 | 6.50 | 0.12 | 19 |
| 87 | 6.5 ± 0.6 | 4.21 | 3.70 | 2.14 | 0.18 | 1.58 | −294 | 2.90 | 0.15 | 9 |
| 115 | 2.8 ± 2.5 | 3.38 | 3.56 | 1.89 | 0.25 | 1.55 | −503 | 0.19 | 0.05 | 20 |
Horizontal tics in Fig. 5b indicate the layer thickness L of the ordered structure near the IL surface. For z ≤ L, the envelope ϕ(z) of the density profile is given by eqn (7), whereas for z > L the envelope is exponentially decaying with the bulk correlation length ξb determined by X-ray scattering.67
Fig. 7 shows the layer thickness L/d of the normalized surface structure divided by its periodicity d vs. reduced temperature τ (red circles). The blue line is a fit to the theoretically predicted growth law assuming exponentially decaying short range interactions (eqn (10a)). Approaching the bulk transition temperature T0, the surface layer thickness L is diverging. From the fit we obtain T0 = 68.2 °C.
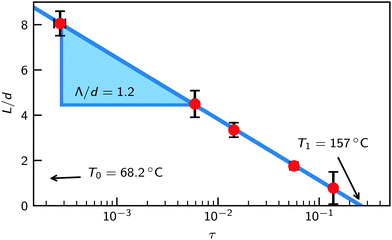 | ||
| Fig. 7 Smectic wetting layer thickness L from fits (red circles) and fit to eqn (10a) (blue line) vs. reduced temperature τ = (T − T0)/T0. | ||
This value agrees well with the transition temperature TLC = 67.1 °C of the metastable smectic bulk phase determined from DSC measurements. On the other hand, surface induced ordering is found only below τ = 0.26. This corresponds to an onset temperature T1 = 157 °C. Above this temperature, the oscillatory structure near interfaces decays exponentially with a decay length given by the corresponding bulk correlation length ξb.91
For the structurally similar imidazolium based IL [C18C1im]+[FAP]− no such surface ordered structures were found in a previous XRR study.55 In contrast, over the entire accessible temperature range the decay length at the surface was found to be equal to the bulk correlation length. However, unlike for [C22C1im]+[NTf2]− no indications for metastable liquid crystalline mesophases have been found in this system.
In literature, at least three distinct types of interface induced smectic order are discussed: discrete, continuous, and continuous with a prewetting layer. Discrete interface induced smectic order has been found for several liquid crystals at the free surface,92–94 in pores,95 and by simulation.96 In these systems, surface freezing via a quantitized layer by layer growth of a smectic liquid crystal was found above the mesophase transition temperature. In contrast, continuous smectic order is characterized by a gradually decaying oscillating density profile. Notably, here the correlation length at the interface is larger than in the isotropic bulk. Several XRR studies,94,97–100 atomic-force microscopy101 and simulations102 show evidence for this phenomena. Surface induced continuous smectic order with prewetting is a mixture of the two cases discussed above. Here, a single smectic monolayer is wetting the surface. Below this first layer, similar to the continuous case an oscillating decaying profile is observed.94
However, these different varieties of interface induced smectic order phenomena can be described within microscopic wetting theory103,104 based on smectic order parameter profiles. There, the film growth of undersaturated phases upon approaching two phase coexistence is studied, and phenomena like layering transitions or prewetting can occur. Depending on the intermolecular interaction potentials, phase transition entropies, and temperature one or the other of structures discussed above is realized.
The first category are liquid crystals that exhibit continuous or quasi-continuous bulk phase transitions, e.g., the commonly used 8-CB and 8-OCB with nematic-smectic transition.100 These transitions are characterized by a vanishing or negligible transition entropy ΔS.87 Most of the previously studied LC systems fall in this category. In these systems, wetting theory predicts pinning of the smectic fluctuations at the surface of the nematic bulk. For continuous phase transitions, Ginzburg–Landau theory predicts that the correlation length ξs of the smectic fluctuations vs. reduced temperature τ scale with a power law
| ξs(τ) = ξ0τ−ν | (11) |
By contrast, at the isotropic-smectic transition of [C22C1im]+[NTf2]− we find ΔSLC = 2.5 mol−1 K−1, which signals a first-order bulk phase transition. From the observed logarithmic growth law for the layer thickness L(τ) one infers complete wetting and effectively short-ranged interactions. Hence, in [C22C1im]+[NTf2]− the surface-induced smectic layer thickness L reaches values that are substantially larger than its bulk correlation length ξb.
Ultimately, the fundamental parameter connecting the system temperature with the surface structure is the pre-factor Λ in the growth law given by eqn (9c). According to theory, this parameter is linked to the bulk correlation length ξb in the isotropic phase.83,103,104 Indeed, we find a very good agreement of Λ/d = 1.2 with the bulk value ξb/d = 1.3 determined by SAXS.67
A similar behavior is expected for other ILs, exhibiting smectic mesophases. However, in equilibrium surface induced smectic order will only be found for systems where T1 > Tm. For example, by extrapolation a transition temperature TLC = −160 °C is predicted for the IL [C14C1im]+[NTf2]− with shorter C14 cation side chains.34 Assuming τ1 < 0.3, surface induced smectic order is only expected to be found below −130 °C. This temperature is about 170 Kelvin below its bulk melting point at 41 °C.
However, for smaller anions such as [BF4] and [PF6] ILC transition temperatures TLC strongly increase. For example, in [C14C1im]+[PF6]− a value of TLC − Tm = 5 K is found.34 Thus, surface induced smectic order might be also relevant for ILs composed of commonly used cations.
5 Conclusions
At the free [C22C1im]+[NTf2]− surface, pronounced ordering was observed. Oscillatory surface profiles give rise to strong quasi-Bragg peaks in the XRR patterns. Quantitative analysis by fitting of model profiles shows that close to the melting point a smectic surface layer of thickness L is wetting the isotropic bulk liquid. These findings are in conclusively agreement with MS-GS and MD simulations, showing smectic-like layering for some IL interfaces.43,105Fig. 8 shows a schematic representation of the surface structures at 68 °C and 115 °C. Notably, close to the melting point we find smectic layer thicknesses up to 30 nm. This corresponds to an ordered surface structure, extending 8 times the smectic periodicity d below the liquid surface. Therefore, unlike for other ILs such as [C18C1im]+[FAP]−, the oscillatory near surface structure persists far beyond the bulk correlation length of ξb/d = 1.3.
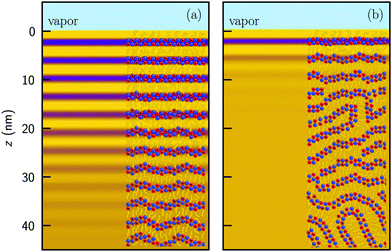 | ||
| Fig. 8 Electron density profiles and schematic representation of the near surface structure at (a) 68 °C and (b) 115 °C. The size of anions (blue), imidazolium rings (red) and aliphatic side chains (yellow) are not to scale. Profiles are identical to Fig. 5b. The in-plane order (not shown) remains liquid-like for all temperatures. | ||
The ordered surface layer thickness L vs. temperature is quantitatively described by a logarithmic growth law. This dependency follows the prediction from a generic thermodynamic model for interface induced phase transitions. In agreement with theory, the pre-factor Λ in the logarithmic growth law agrees with the bulk correlation length ξb of the isotropic liquid phase. Upon cooling, the smectic layer thickness L/d in [C22C1im]+[NTf2]− diverges at a temperature T0. Interestingly, T0 agrees well with the transition temperature TLC of a metastable liquid crystalline smectic SmA2-mesophase formed from the supercooled isotropic liquid. This indicates that the observed layered surface structure in [C22C1im]+[NTf2]− is governed by surface induced smectic order, i.e. fluctuations into the metastable SmA2-phase. On the other hand, we find an upper transition temperature of T1 = 157 °C for the ordered surface layer. Note that this behavior is very different from the power law scaling found for LCs with quasi-continuous nematic-smectic phase transitions.97,100 In contrast to other soft matter systems such as liquid crystals or alkanes, surface induced smectic ordering in the ionic liquid [C22C1im]+[NTf2]− is relevant over a much larger temperatures range.
In bulk, smectic mesophases were found for a wide class of neat and mixed ILs.19 Based on our results, we therefore presume that similar surface structures are also formed in other ILs. From our previous bulk67 and surface studies,55 we anticipate that the tendency for surface induced smectic order is larger for smaller anions and longer aliphatic side chains. This tendency is supported by clear trends observed in a new volume based approach to predict the thermodynamical behavior of ILs and ILCs.34
The well ordered surface structures observed for [C22C1im]+[NTf2]− over a surprisingly large temperature range might significantly reduce the diffusion of guest molecules across an IL/gas interface. Indeed, for [C16C1im][NO3] MD simulations show 5-fold smaller diffusion constants perpendicular to the layers of the smectic phase.106 Formation of such diffusion barriers for reactants and products, might adversely affect the performance of ILs in SILP processes.8 Further experiments on different ILs will be required to shed light on the complex interplay between the molecular structure of the anions and cations and the tendency for surface induced smectic order.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Grazing incidence surface scattering and preliminary XRR data were obtained at ID10-EH1, ESRF The European Synchrotron, Grenoble, France. XRR experiments were conducted at the LISA instrument at beamline P08, Petra III, DESY, Hamburg, Germany funded by BMBF (project 05K10FK2 and 05K130FK2). We thank Gunnar Kircher (MPI-P) for [C22C1im]+[NTf2]− synthesis, Xilin Wu (MPI-P) and Guoming Liu (Chin. Acad. Sci.) for assistance during the synchrotron experiments and Peter Reichert (MPI-P), Julia Haddad (Bar-Illan Univ.), and Hans-Jürgen Butt (MPI-P) for helpful discussions. J. M. and M. M. acknowledge the MAINZ Graduate School of Excellence, funded through the Excellence Initiative (DFG/GSC 266) and the German Israeli Foundation for scientific research and development (2306-2310.14/2011) for financial support. B. H. received a scholarship funded by the ESRF The European Synchrotron, Grenoble, France. H. L. was supported by the China Scholarship Council. Open Access funding provided by the Max Planck Society.References
- L. Crowhurst, N. L. Lancaster, J. M. Pérez Arlandis and T. Welton, J. Am. Chem. Soc., 2004, 126, 11549–11555 CrossRef CAS PubMed.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- J. S. Wilkes, J. A. Levisky, R. A. Wilson and C. L. Hussey, Inorg. Chem., 1982, 21, 1263–1264 CrossRef CAS.
- W. Xu and C. A. Angell, Science, 2003, 302, 422–425 CrossRef CAS PubMed.
- A. Riisager, P. Wasserscheid, R. van Hal and R. Fehrmann, J. Catal., 2003, 219, 452–455 CrossRef CAS.
- R. Fehrmann, A. Riisager and M. Haumann, Supported ionic liquids: fundamentals and applications, John Wiley & Sons, 2013 Search PubMed.
- H.-P. Steinrueck and P. Wasserscheid, Catal. Lett., 2015, 145, 380–397 CrossRef CAS.
- K. R. Lovelock, Phys. Chem. Chem. Phys., 2012, 14, 5071–5089 RSC.
- A. Bradley, C. Hardacre, J. Holbrey, S. Johnston, S. McMath and M. Nieuwenhuyzen, Chem. Mater., 2002, 14, 629–635 CrossRef CAS.
- J. De Roche, C. M. Gordon, C. T. Imrie, M. D. Ingram, A. R. Kennedy, F. Lo Celso and A. Triolo, Chem. Mater., 2003, 15, 3089–3097 CrossRef CAS.
- A. Triolo, A. Mandanici, O. Russina, V. Rodriguez-Mora, M. Cutroni, C. Hardacre, M. Nieuwenhuyzen, H.-J. Bleif, L. Keller and M. A. Ramos, J. Phys. Chem. B, 2006, 110, 21357–21364 CrossRef CAS PubMed.
- A. Triolo, O. Russina, H.-J. Bleif and E. Di Cola, J. Phys. Chem. B, 2007, 111, 4641–4644 CrossRef CAS PubMed.
- K. Fujii, R. Kanzaki, T. Takamuku, Y. Kameda, S. Kohara, M. Kanakubo, M. Shibayama, S.-I. Ishiguro and Y. Umebayashi, J. Chem. Phys., 2011, 135, 244502 CrossRef PubMed.
- C. S. Santos, N. S. Murthy, G. A. Baker and E. W. Castner Jr, J. Chem. Phys., 2011, 134, 121101 CrossRef PubMed.
- H. K. Kashyap, J. J. Hettige, H. V. Annapureddy and C. J. Margulis, Chem. Commun., 2012, 48, 5103–5105 RSC.
- H. K. Kashyap, C. S. Santos, H. V. Annapureddy, N. S. Murthy, C. J. Margulis and E. W. Castner Jr, Faraday Discuss., 2012, 154, 133–143 RSC.
- R. Caminiti and L. Gontrani, The structure of ionic liquids, Springer, 2014, vol. 193 Search PubMed.
- K. Fujii, S. Kohara and Y. Umebayashi, Phys. Chem. Chem. Phys., 2015, 17, 17838–17843 RSC.
- F. Nemoto, M. Kofu and O. Yamamuro, J. Phys. Chem. B, 2015, 119, 5028–5034 CrossRef CAS PubMed.
- C. Hardacre, J. D. Holbrey, C. L. Mullan, T. G. Youngs and D. T. Bowron, J. Chem. Phys., 2010, 133, 074510 CrossRef PubMed.
- S. Shigeto and H.-o. Hamaguchi, Chem. Phys. Lett., 2006, 427, 329–332 CrossRef CAS.
- C. Margulis, Mol. Phys., 2004, 102, 829–838 CrossRef CAS.
- A. A. Pádua, M. F. Costa Gomes and J. N. Canongia Lopes, Acc. Chem. Res., 2007, 40, 1087–1096 CrossRef PubMed.
- J. N. Canongia Lopes and A. A. Pádua, J. Phys. Chem. B, 2006, 110, 3330–3335 Search PubMed.
- H. V. Annapureddy, H. K. Kashyap, P. M. De Biase and C. J. Margulis, J. Phys. Chem. B, 2010, 114, 16838–16846 CrossRef CAS PubMed.
- K. Shimizu, M. F. C. Gomes, A. A. Pádua, L. P. Rebelo and J. N. C. Lopes, THEOCHEM, 2010, 946, 70–76 CrossRef CAS.
- J. J. Hettige, H. K. Kashyap, H. V. Annapureddy and C. J. Margulis, J. Phys. Chem. Lett., 2012, 4, 105–110 CrossRef PubMed.
- K. Shimizu, C. E. Bernardes and J. N. Canongia Lopes, J. Phys. Chem. B, 2014, 118, 567–576 CrossRef CAS PubMed.
- J. C. Araque, J. J. Hettige and C. J. Margulis, J. Phys. Chem. B, 2015, 119, 12727–12740 CrossRef CAS PubMed.
- C. J. Bowlas, D. W. Bruce and K. R. Seddon, Chem. Commun., 1996, 1625–1626 RSC.
- C. M. Gordon, J. D. Holbrey, A. R. Kennedy and K. R. Seddon, J. Mater. Chem., 1998, 8, 2627–2636 RSC.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133–2140 RSC.
- C. Hardacre, J. D. Holbrey, P. B. McCormac, S. J. McMath, M. Nieuwenhuyzen and K. R. Seddon, J. Mater. Chem., 2001, 11, 346–350 RSC.
- Y. V. Nelyubina, A. S. Shaplov, E. I. Lozinskaya, M. I. Buzin and Y. S. Vygodskii, J. Am. Chem. Soc., 2016, 138, 10076–10079 CrossRef CAS PubMed.
- Y. Ji, R. Shi, Y. Wang and G. Saielli, J. Phys. Chem. B, 2013, 117, 1104–1109 CrossRef CAS PubMed.
- Y. Wang and G. A. Voth, J. Am. Chem. Soc., 2005, 127, 12192–12193 CrossRef CAS PubMed.
- Y. Wang and G. A. Voth, J. Phys. Chem. B, 2006, 110, 18601–18608 CrossRef CAS PubMed.
- G. Saielli, Soft Matter, 2012, 8, 10279–10287 RSC.
- G. Saielli, A. Bagno and Y. Wang, J. Phys. Chem. B, 2015, 119, 3829–3836 CrossRef CAS PubMed.
- G. Saielli and Y. Wang, J. Phys. Chem. B, 2016, 120, 9152–9160 CrossRef CAS PubMed.
- R. Lynden-Bell and M. Del Popolo, Phys. Chem. Chem. Phys., 2006, 8, 949–954 RSC.
- R. M. Lynden-Bell, M. G. Del Pópolo, T. G. Youngs, J. Kohanoff, C. G. Hanke, J. B. Harper and C. C. Pinilla, Acc. Chem. Res., 2007, 40, 1138–1145 CrossRef CAS PubMed.
- W. D. Amith, J. J. Hettige, E. W. Castner and C. J. Margulis, J. Phys. Chem. Lett., 2016, 3785–3790 CrossRef CAS PubMed.
- C. Kolbeck, T. Cremer, K. Lovelock, N. Paape, P. Schulz, P. Wasserscheid, F. Maier and H.-P. Steinruck, J. Phys. Chem. B, 2009, 113, 8682–8688 CrossRef CAS PubMed.
- F. Maier, T. Cremer, C. Kolbeck, K. Lovelock, N. Paape, P. Schulz, P. Wasserscheid and H.-P. Steinrück, Phys. Chem. Chem. Phys., 2010, 12, 1905–1915 RSC.
- V. Lockett, R. Sedev, S. Harmer, J. Ralston, M. Horne and T. Rodopoulos, Phys. Chem. Chem. Phys., 2010, 12, 13816–13827 RSC.
- K. R. Lovelock, I. J. Villar-Garcia, F. Maier, H.-P. SteinruÌĹck and P. Licence, Chem. Rev., 2010, 110, 5158–5190 CrossRef CAS PubMed.
- C. Kolbeck, A. Deyko, T. Matsuda, F. T. Kohler, P. Wasserscheid, F. Maier and H.-P. Steinrück, ChemPhysChem, 2013, 14, 3726–3730 CrossRef CAS PubMed.
- C. Kolbeck, I. Niedermaier, A. Deyko, K. R. Lovelock, N. Taccardi, W. Wei, P. Wasserscheid, F. Maier and H.-P. Steinrück, Chem. – Eur. J., 2014, 20, 3954–3965 CrossRef CAS PubMed.
- S. Rivera-Rubero and S. Baldelli, J. Phys. Chem. B, 2006, 110, 4756–4765 CrossRef CAS PubMed.
- C. S. Santos and S. Baldelli, J. Phys. Chem. B, 2009, 113, 923–933 CrossRef CAS PubMed.
- E. Sloutskin, B. M. Ocko, L. Tamam, I. Kuzmenko, T. Gog and M. Deutsch, J. Am. Chem. Soc., 2005, 127, 7796–7804 CrossRef PubMed.
- N. Nishi, Y. Yasui, T. Uruga, H. Tanida, T. Yamada, S.-I. Nakayama, H. Matsuoka and T. Kakiuchi, J. Chem. Phys., 2010, 132, 164705 CrossRef PubMed.
- N. Nishi, T. Uruga, H. Tanida and T. Kakiuchi, Langmuir, 2011, 27, 7531–7536 CrossRef CAS PubMed.
- M. Mezger, B. M. Ocko, H. Reichert and M. Deutsch, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3733–3737 CrossRef CAS PubMed.
- M. Mezger, H. Schröder, H. Reichert, S. Schramm, J. S. Okasinski, S. Schöder, V. Honkimäki, M. Deutsch, B. M. Ocko and J. Ralston, Science, 2008, 322, 424–428 CrossRef CAS PubMed.
- M. Mezger, S. Schramm, H. Schröder, H. Reichert, M. Deutsch, E. J. De Souza, J. S. Okasinski, B. M. Ocko, V. Honkimäki and H. Dosch, J. Chem. Phys., 2009, 131, 094701 CrossRef PubMed.
- Z. Brkljača, M. Klimczak, Z. Miličević, M. Weisser, N. Taccardi, P. Wasserscheid, D. M. Smith, A. Magerl and A.-S. Smith, J. Phys. Chem. Lett., 2015, 6, 549–555 CrossRef PubMed.
- T. A. Petach, A. Mehta, R. Marks, B. Johnson, M. F. Toney and D. Goldhaber-Gordon, ACS Nano, 2016, 10, 4565–4569 CrossRef CAS PubMed.
- Y. Jeon, D. Vaknin, W. Bu, J. Sung, Y. Ouchi, W. Sung and D. Kim, Phys. Rev. Lett., 2012, 108, 055502 CrossRef PubMed.
- L. A. Jurado, H. Kim, A. Arcifa, A. Rossi, C. Leal, N. D. Spencer and R. M. Espinosa-Marzal, Phys. Chem. Chem. Phys., 2015, 17, 13613–13624 RSC.
- R. S. Anaredy and S. K. Shaw, Langmuir, 2016, 5147–5154 CrossRef CAS PubMed.
- T. Li, F. Xu and W. Shi, Chem. Phys. Lett., 2015, 628, 9–15 CrossRef CAS.
- E. F. G. Herington, Zone melting of organic compounds, Wiley, 1963 Search PubMed.
- W. R. Wilcox, R. Friedenberg and N. Back, Chem. Rev., 1964, 64, 187–220 CrossRef CAS.
- A. R. Choudhury, N. Winterton, A. Steiner, A. I. Cooper and K. A. Johnson, J. Am. Chem. Soc., 2005, 127, 16792–16793 CrossRef CAS PubMed.
- H. Weiss, J. Mars, H. Li, G. Kircher, O. Ivanova, A. Feoktystov, O. Soltwedel, M. Bier and M. Mezger, J. Phys. Chem. B, 2017, 121, 620–629 CrossRef CAS PubMed.
- B. M. Murphy, M. Greve, B. Runge, C. T. Koops, A. Elsen, J. Stettner, O. H. Seeck and O. M. Magnussen, J. Synchrotron Radiat., 2014, 21, 45–56 CrossRef CAS PubMed.
- B. L. Henke, E. M. Gullikson and J. C. Davis, At. Data Nucl. Data Tables, 1993, 54, 181–342 CrossRef CAS.
- C. T. Chantler, J. Phys. Chem. Ref. Data, 1995, 24, 71–643 CrossRef CAS.
- C. T. Chantler, J. Phys. Chem. Ref. Data, 2000, 29, 597–1056 CrossRef CAS.
- M. Tolan, X-ray scattering from soft-matter thin films: materials science and basic research, Springer Berlin, 1999 Search PubMed.
- J. Als-Nielsen and D. McMorrow, Elements of modern X-ray physics, Wiley, 2011 Search PubMed.
- K.-M. Zimmermann, M. Tolan, R. Weber, J. Stettner, A. Doerr and W. Press, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 10377 CrossRef CAS.
- T. Hohage, K. Giewekemeyer and T. Salditt, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 051604 CrossRef PubMed.
- L. Nevot and P. Croce, Rev. Phys. Appl., 1980, 15, 761–779 CrossRef CAS.
- P. de Gennes and J. Prost, The physics of liquid crystals, Oxford, 1998 Search PubMed.
- L. G. Parratt, Phys. Rev., 1954, 95, 359 CrossRef.
- L. Ingber, Math. Comput. Modell., 1993, 18, 29–57 CrossRef.
- A. Braslau, P. S. Pershan, G. Swislow, B. Ocko and J. Als-Nielsen, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 2457 CrossRef.
- M. Sanyal, S. Sinha, K. Huang and B. Ocko, Phys. Rev. Lett., 1991, 66, 628 CrossRef CAS PubMed.
- C. Kolbeck, J. Lehmann, K. Lovelock, T. Cremer, N. Paape, P. Wasserscheid, A. Froba, F. Maier and H.-P. Steinruck, J. Phys. Chem. B, 2010, 114, 17025–17036 CrossRef CAS PubMed.
- R. Lipowsky, Phys. Rev. Lett., 1982, 49, 1575 CrossRef CAS.
- J. F. Van Der Veen, Surf. Sci., 1999, 433, 1–11 CrossRef.
- Y. Yang, M. Asta and B. B. Laird, Phys. Rev. Lett., 2013, 110, 096102 CrossRef PubMed.
- B. Ocko, X. Wu, E. Sirota, S. Sinha, O. Gang and M. Deutsch, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1997, 55, 3164 CrossRef CAS.
- W. E. Acree Jr and J. S. Chickos, J. Phys. Chem. Ref. Data, 2006, 35, 1051–1330 CrossRef.
- I. Dierking, Textures of liquid crystals, John Wiley & Sons, 2003 Search PubMed.
- P. S. Pershan and M. Schlossman, Liquid Surfaces and Interfaces: Synchrotron X-ray Methods, Cambridge University Press, 2012 Search PubMed.
- J. Als-Nielsen, D. Jacquemain, K. Kjaer, F. Leveiller, M. Lahav and L. Leiserowitz, Phys. Rep., 1994, 246, 251–313 CrossRef CAS.
- M. Mezger, R. Roth, H. Schröder, P. Reichert, D. Pontoni and H. Reichert, J. Chem. Phys., 2015, 142, 164707 CrossRef PubMed.
- B. Ocko, A. Braslau, P. S. Pershan, J. Als-Nielsen and M. Deutsch, Phys. Rev. Lett., 1986, 57, 94 CrossRef CAS PubMed.
- B. Ocko, Phys. Rev. Lett., 1990, 64, 2160 CrossRef CAS PubMed.
- R. Lucht, P. Marczuk, C. Bahr and G. Findenegg, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2001, 63, 041704 CrossRef CAS PubMed.
- G. S. Iannacchione, J. T. Mang, S. Kumar and D. Finotello, Phys. Rev. Lett., 1994, 73, 2708 CrossRef CAS PubMed.
- J. V. Selinger and D. R. Nelson, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 37, 1736 CrossRef.
- J. Als-Nielsen, F. Christensen and P. S. Pershan, Phys. Rev. Lett., 1982, 48, 1107 CrossRef CAS.
- P. S. Pershan and J. Als-Nielsen, Phys. Rev. Lett., 1984, 52, 759 CrossRef CAS.
- P. S. Pershan, A. Braslau, A. Weiss and J. Als-Nielsen, Phys. Rev. A: At., Mol., Opt. Phys., 1987, 35, 4800 CrossRef CAS.
- M. Fukuto, O. Gang, K. J. Alvine, B. M. Ocko and P. S. Pershan, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 031607 CrossRef PubMed.
- K. Kočevar and I. Muševič, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 65, 021703 CrossRef PubMed.
- L. Mederos and D. Sullivan, Phys. Rev. A: At., Mol., Opt. Phys., 1992, 46, 7700 CrossRef.
- S. Dietrich, Phase Transitions and Critical Phenomena, 1988, pp. 1–218 Search PubMed.
- M. Schick, Liquids at Interfaces, Les Houches Session XLVIII, 1988, pp. 415–497 Search PubMed.
- W. Jiang, Y. Wang, T. Yan and G. A. Voth, J. Phys. Chem. C, 2008, 112, 1132–1139 CAS.
- G. Saielli, G. A. Voth and Y. Wang, Soft Matter, 2013, 9, 5716–5725 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cp04852a |
| ‡ Present address: Department of Chemistry and Physical Science, School of Natural and Social Sciences, Mount Vernon Nazarene University, 800 Martinsburg Road, Mount Vernon, Ohio 43050, USA. |
| This journal is © the Owner Societies 2017 |