Two-step volume phase transition mechanism of poly(N-vinylcaprolactam) hydrogel online-tracked by two-dimensional correlation spectroscopy†
Abstract
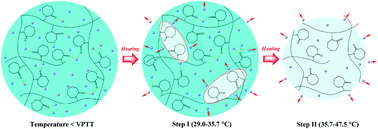
In this study, temperature-dependent FTIR spectroscopy in combination with the perturbation–correlation moving-window (PCMW2D) technique and generalized two-dimensional (2D) correlation analysis was applied to investigate the phase transition mechanism of poly(N-vinylcaprolactam) (PVCL) hydrogel upon heating. In the conventional 1D FTIR spectra, the gradual dehydration of C–H groups, as well as the gradual dissociation of hydrogen bonds between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and water molecules, was observed during phase transition. Moreover, we found that the rate at which water molecules were expelled out of the gel network during phase transition was changed to a sigmoid mode, rather than increasing linearly with increasing temperature. PCMW2D FTIR spectra revealed that the phase transition of PVCL hydrogel can be divided into two steps (named as I and II) upon heating, and we further determined the temperature regions of steps I and II to be 29.0–35.7 °C and 35.7–47.5 °C, respectively. Step I is the formation of hydrophobic domains in the gel, and step II is the chain collapse of the gel. Finally, with the help of generalized 2D correlation analysis, it was confirmed that the transformation of hydrogen bonds was the driving force of the hydrophobic domain formation process, while the hydrophobic interaction of C–H groups was the driving force for the chain collapse process. Combined with the obtained sequential orders of step I and step II, an integrated two-step phase transition mechanism of PVCL hydrogel upon heating was proposed.
O groups and water molecules, was observed during phase transition. Moreover, we found that the rate at which water molecules were expelled out of the gel network during phase transition was changed to a sigmoid mode, rather than increasing linearly with increasing temperature. PCMW2D FTIR spectra revealed that the phase transition of PVCL hydrogel can be divided into two steps (named as I and II) upon heating, and we further determined the temperature regions of steps I and II to be 29.0–35.7 °C and 35.7–47.5 °C, respectively. Step I is the formation of hydrophobic domains in the gel, and step II is the chain collapse of the gel. Finally, with the help of generalized 2D correlation analysis, it was confirmed that the transformation of hydrogen bonds was the driving force of the hydrophobic domain formation process, while the hydrophobic interaction of C–H groups was the driving force for the chain collapse process. Combined with the obtained sequential orders of step I and step II, an integrated two-step phase transition mechanism of PVCL hydrogel upon heating was proposed.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...