Computational design of pH-switchable control agents for nitroxide mediated polymerization†
Abstract
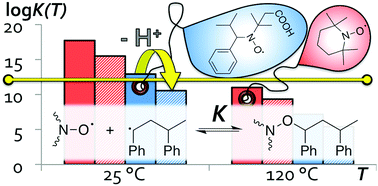
In the present work we use accurate quantum chemistry to evaluate several known and novel nitroxides bearing acid–base groups as pH-switchable control agents for room temperature NMP. Based on G3(MP2,CC)(+)//M06-2X/6-31+G(d) calculations with UAKS-CPCM/M06-2X/6-31+G(d) solvation corrections, a number of novel nitroxides are predicted to be suitable for controlled polymerization of bulk styrene at room temperature when deprotonated (i.e. negatively charged), while remaining inert when neutral. These include an α-ethyl analogue of 3-carboxy-PROXYL and novel derivatives of 2,2,5-trimethyl-4-phenyl-3-azahexane-3-nitroxide (TIPNO) that have been modified to include acidic groups. Among the other species evaluated, 3,4-dicarboxy-PROXYL, α-carboxylated PROXYL and the phosphoric acid derivative of N-(2-methylpropyl)-N-(1-diethylphosphono-2,2-dimethylpropyl)-N-oxyl (SG1) are predicted to undergo suitable pH-switching at around 60 °C, and may also be fitting for some applications.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...