Biomembrane solubilization mechanism by Triton X-100: a computational study of the three stage model†
Abstract
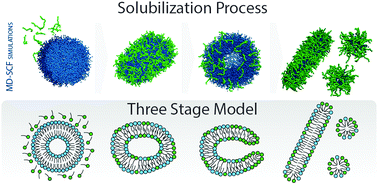
The solubilization mechanism of lipid membranes in the presence of Triton X-100 (TX-100) is investigated at molecular resolution using molecular dynamics (MD) simulations. Thanks to the large time and length scales accessible by the hybrid particle–field formulation of the models employed here, the complex process of membrane solubilization has been studied, with the goal of verifying the three stage model reported in the literature. DPPC lipid bilayers and vesicles have been studied at different concentrations of the TX-100 detergent employing coarse grained (CG) models. Systems up to ∼600.000 beads, corresponding to more than 2 millions heavy atoms, have been simulated. Moreover, in order to clarify several experimental pieces of evidence, both slow and fast detergent partition scenarios have been investigated. Flat and curved (vesicles) lipid bilayer surfaces, interacting with TX-100, have been considered to study the curvature effects on the detergent partition rate in the membrane. Shape and conformational changes of mixed DPPC/TX-100 vesicles, as a function of TX-100 content, have also been studied. In particular, high curvature surfaces, corresponding to a higher local TX-100 content, promote a membrane rupture. In flat lipid surfaces, on the time scale simulated the detergent partition is almost absent, following a different pathway of the solubilization membrane mechanism.


 Please wait while we load your content...
Please wait while we load your content...