Elucidating the mechanism of MgB2 initial hydrogenation via a combined experimental–theoretical study†
Abstract
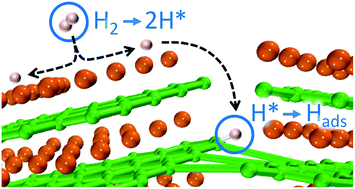
Mg(BH4)2 is a promising solid-state hydrogen storage material, releasing 14.9 wt% hydrogen upon conversion to MgB2. Although several dehydrogenation pathways have been proposed, the hydrogenation process is less well understood. Here, we present a joint experimental–theoretical study that elucidates the key atomistic mechanisms associated with the initial stages of hydrogen uptake within MgB2. Fourier transform infrared, X-ray absorption, and X-ray emission spectroscopies are integrated with spectroscopic simulations to show that hydrogenation can initially proceed via direct conversion of MgB2 to Mg(BH4)2 complexes. The associated energy landscape is mapped by combining ab initio calculations with barriers extracted from the experimental uptake curves, from which a kinetic model is constructed. The results from the kinetic model suggest that initial hydrogenation takes place via a multi-step process: molecular H2 dissociation, likely at Mg-terminated MgB2 surfaces, is followed by migration of atomic hydrogen to defective boron sites, where the formation of stable B–H bonds ultimately leads to the direct creation of Mg(BH4)2 complexes without persistent BxHy intermediates. Implications for understanding the chemical, structural, and electronic changes upon hydrogenation of MgB2 are discussed.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...