Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oxygen storage properties of hexagonal HoMnO3+δ†
Konrad
Świerczek
ab,
Alicja
Klimkowicz
 *c,
Kengo
Nishihara
c,
Shuntaro
Kobayashi
c,
Akito
Takasaki
c,
Maleeha
Alanizy
d,
Stanislaw
Kolesnik
d,
Bogdan
Dabrowski
d,
Seungho
Seong
e and
Jeongsoo
Kang
e
*c,
Kengo
Nishihara
c,
Shuntaro
Kobayashi
c,
Akito
Takasaki
c,
Maleeha
Alanizy
d,
Stanislaw
Kolesnik
d,
Bogdan
Dabrowski
d,
Seungho
Seong
e and
Jeongsoo
Kang
e
aAGH University of Science and Technology, Faculty of Energy and Fuels, Department of Hydrogen Energy, al. A. Mickiewicza 30, 30-059 Krakow, Poland
bAGH Centre of Energy, AGH University of Science and Technology, ul. Czarnowiejska 36, 30-054 Krakow, Poland
cShibaura Institute of Technology, Department of Engineering Science and Mechanics, 3-7-5 Toyosu, Koto-ku, 135-8548 Tokyo, Japan. E-mail: klimkowicz.alicja.ewa.j2@shibaura-it.ac.jp
dDepartment of Physics, Northern Illinois University, DeKalb, IL 60115, USA
eDepartment of Physics, The Catholic University of Korea (CUK), Bucheon 14662, Korea
First published on 12th July 2017
Abstract
Structural and oxygen content changes of hexagonal HoMnO3+δ manganite at the stability boundary in the perovskite phase have been studied by X-ray diffraction and thermogravimetry using in situ oxidation and reduction processes at elevated temperatures in oxygen and air. The oxygen storage properties during structural transformation between stoichiometric Hex0 and oxygen-loaded Hex1 phases, transition temperatures and kinetics of the oxygen incorporation and release are reported for materials prepared by the solid-state synthesis and high-impact mechanical milling. Long-term annealing experiments have shown that the Hex0 (δ = 0) → Hex1 (δ ≈ 0.28) phase transition is limited by the surface reaction and nucleation of the new phase for HoMnO3+δ 15MM. The temperatures of Hex0 ↔ Hex1 transitions have been established at 290 °C and 250 °C upon heating and cooling, respectively, at a rate of 0.1° min−1, also indicating that the temperature hysteresis of the transition could possibly be as small as 10 °C in the equilibrium. Ball-milling of HoMnO3+δ has only a small effect on improving the speed of the reduction/oxidation processes in oxygen, but importantly, allowed for considerable oxygen incorporation in air at a temperature range of 220–255 °C after prolonged heating. The Mn 2p XAS results of the Mn valence in oxygen loaded samples support the oxygen content determined by the TG method. The magnetic susceptibility data of the effective Mn valence gave inconclusive results due to dominating magnetism of the Ho3+ ions. Comparison of HoMnO3+δ with previously studied DyMnO3+δ indicates that a tiny increase in the ionic size of lanthanide has a huge effect on the redox properties of hexagonal manganites and that practical properties could be significantly improved by synthesizing the larger average size (Y,Ln)MnO3+δ manganites.
1. Introduction
Usage of pure or highly enriched oxygen gas is nowadays ubiquitous in various industrial technologies because of increased efficiency and/or the uniqueness of chemical processes requiring it. Enriched oxygen gas is essential, among others, in the production processes of steel and non-ferrous metals, chemicals, petrochemicals, glass, ceramics, and paper, and also it is utilized in healthcare and medical treatments.1 At a large scale, the oxygen is produced by cryogenic methods, which involve large capital investment, require specific conditions of pressure and temperature, and consequently, are considered highly energy-consuming and costly.2 Alternative methods rely on the separation of air components via pressure- and/or temperature-driven adsorption processes3,4 and the emerging membrane-related technologies.5,6Interestingly, with the recent progress in the field of so-called oxygen storage materials (OSMs), several novel compounds appear to be capable of economical production of oxygen by using thermal or pressure swing-type reactions.7–11 Moreover, depending on their intrinsic properties, these OSMs are also considered for implementation in many important existing and emerging technological processes: for example, inert gas purification; solar water splitting; non-aerobic oxidation including flameless combustion (e.g., synthesis gas production); high-temperature production of steel, glass or plastic production that requires high-purity oxygen; oxy-fuel and chemical looping combustion processes; solid oxide fuel cell technology and the three-way catalytic converters for automotive exhaust systems.12–21
The underlying basis of oxygen incorporation/release into/from OSMs is associated with a large change of the oxygen stoichiometry in the bulk of the material, which is essentially a redox-type process.10,11,21 For example, as reported by Motohashi et al.10 for the A-site layer-ordered perovskite system, BaYMn2O5+δ, a reversible change in the oxygen content between O5 (δ = 0) and O6 (δ = 1) compositions (3.85 wt% theoretical change) is achievable during an oxygen partial pressure swing process (by reduction in 5 vol% H2/Ar and oxidation in air) at 500 °C. The reduction time of the powdered BaYMn2O6 sample was shown to be in the order of minutes at 500 °C, with a practical oxygen storage capacity (OSC) exceeding 3.7 wt%.11 Oxidation performed in air at the same temperature proceeded much faster (seconds), which was mainly caused by an exothermic reaction (∼200 kJ mol−1) associated with oxygen incorporation.22 Consequently, it was found that the reduction process is a limiting factor for maximizing the OSC of such compounds.
Partial substitution of selected lanthanides at the Y-site increased reduction rates, for instance, for BaY0.75Pr0.25Mn2O5+δ compound, the OSC remained above 3.5 wt%.23 In another approach, Motohashi et al. showed that BaYMn2O6 powder with an increased specific surface area exhibits faster oxygen release and intake rates.24 Similar enhancement of the oxygen exchange rates by high-energy mechanical milling was also recently reported for BaPrMn2O5+δ and BaSmMn2O5+δ materials.25
Alternative to materials using the oxygen partial pressure swing process, the hexagonal-type manganites LnMnO3+δ (Ln = smaller lanthanides, e.g. Dy, Ho, Er and Y) were found to operate in the much more promising temperature swing process in pure oxygen or air, as recently documented by Remsen et al.7 and Abughayada et al.8,26 The advantage of such compounds stems from a low and narrow temperature range at which the oxygen exchanges occur, and most importantly, there is no need to use special gas mixtures for the reduction process. However, the kinetics of the redox processes reported to date have been relatively slow, and the achievable OSC has been somewhat lower than that for the layer-ordered perovskites.9,23 The substitutions and processing related improvements of redox properties observed for layered perovskites suggest that similar improvements should be possible for hexagonal manganites.
The LnMnO3±δ manganites are known to exhibit the perovskite- or hexagonal-type crystal structure depending on the size of the lanthanide and, to some degree, on the oxygen content during the synthesis.26,27 For larger lanthanides (La–Dy), the distorted perovskite structure is observed when synthesized in air. For smaller ones (Ho–Lu, In and Sc), the hexagonal structure (Hex0, δ = 0) is formed with layers of 8-fold coordinated Ln3+ cations and layers of the corner-shared MnO5 trigonal-bipyramids.8,26 This five-fold coordination of Mn3+ causes splitting of the 3d4 electronic orbitals into three sets: empty a′ (dz2) and filled up e′ (dx2−y2, dxy) and e′′ (dxz, dyz) with high spin states e′2 and e′′2. As a result of orbital filling, the elongation of basal Mn–O bonds and the compression of the apical Mn–O bond are observed. Interestingly, it was shown that the stable hexagonal phase can be converted to the perovskite one by special treatments, e.g., high pressure,28,29 deposition of strained thin films30 or by using soft chemistry methods, followed by appropriate annealing.31 The reverse conversion of the perovskite to the hexagonal structure under reducing conditions was also documented for DyMnO3.7
Until the recent reports by Remsen et al. regarding DyMnO3+δ and substituted Dy1−xYxMnO3+δ,7,31 not much was known about the oxygen hyperstoichiometry in hexagonal manganites. The subsequent paper focused on the structural, magnetic and oxygen storage-related properties of the Dy1−xYxMnO3+δ series,26 in which the superstructure R3c (tripled along the c-axis, Hex1) of Dy0.7Y0.3MnO3.29 was reported. Furthermore, similar behavior was also observed for the hexagonal HoMnO3+δ, ErMnO3+δ, and YMnO3+δ, obtained by high oxygen pressure annealing (Hex2, δ ≈ 0.41) for which OSC, as well as the temperatures of oxidation and reduction, were reported depending on the ionic radius of R3+.8 However, because the observed kinetics of the oxidation/reduction processes were very slow, the determination of the respective phase diagrams of stability of the stoichiometric and oxygen-loaded phases was not complete. Moreover, such a slow kinetics was responsible for the reduced OSC, which could hinder applicability of hexagonal LnMnO3+δ manganites for use at elevated temperatures where waste heat is frequently available from several industrial processes.
In this work, we report on the structural and oxygen content changes of the selected HoMnO3+δ materials at the stability boundary in air of the hexagonal phase recorded during long-time in situ oxidation and reduction at elevated temperatures and in constant oxygen partial pressure conditions of pure oxygen and air. Despite a very small difference in the ionic sizes of Ho and Dy1−xYxMnO3+δ, a significant difference in redox reactions is observed. Synthesis and processing methods have a large effect on the properties as well. A discussion pertaining to the size of substituted Ln cation and the sample preparation method is given on the oxygen storage-related properties, such as the temperature and speed of the oxygen intake and release, the oxygen storage capacity, and the phase stability of the materials. It is shown that enhancement of the OSC characteristics can be achieved by high-impact ball-milling, which produces powders with an increased specific surface area. The valence states of Mn and Ho ions in HoMnO3+δ were determined by employing soft X-ray absorption spectroscopy (XAS), where synchrotron radiation is used as the excitation photon source. XAS is known to be a powerful experimental tool for studying the electronic structures of solids,32,33 such as the valence and spin states of the constituent transition-metal and rare-earth ions.
2. Experimental procedures
2.1 Material preparation
The reference Hex0 hexagonal HoMnO3 0MM (non-milled) samples for in situ X-ray and thermogravimetric measurements were synthesized by a typical high-temperature ceramic method from MnO2 and Ho2O3 oxides. Stoichiometric proportions of the oxides were mechanically milled under dry conditions using zirconia balls to powder at a ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pressed into pellets and, to obtain small grain samples, sintered at a low temperature of 1050 °C for 8 h in air followed by fast cooling to room temperature (RT). A part of the sample, denoted as 15MM, was additionally subjected to high-energy milling, conducted using a Fritsch planetary ball mill, model P7, for 15 minutes, with a rotational speed of 400 rpm. An additional set of large-sized grains of HoMnO3 0MM samples using X-ray absorption spectroscopy and magnetic measurements was obtained with the precisely defined oxygen contents by utilizing information obtained from the in situ X-ray and thermogravimetric measurements, 3.00 (Hex0, as synthesized in air at 1400 °C followed by fast cooling to RT), 3.39 (Hex2, obtained by high-pressure oxygen annealing under 215 bar of pure oxygen at 400 °C followed by slow cooling of the as-made sample to RT) and 3.28 (Hex1, obtained by annealing in oxygen at 270 °C followed by fast cooling to RT of the high pressure oxygen loaded sample).
1, pressed into pellets and, to obtain small grain samples, sintered at a low temperature of 1050 °C for 8 h in air followed by fast cooling to room temperature (RT). A part of the sample, denoted as 15MM, was additionally subjected to high-energy milling, conducted using a Fritsch planetary ball mill, model P7, for 15 minutes, with a rotational speed of 400 rpm. An additional set of large-sized grains of HoMnO3 0MM samples using X-ray absorption spectroscopy and magnetic measurements was obtained with the precisely defined oxygen contents by utilizing information obtained from the in situ X-ray and thermogravimetric measurements, 3.00 (Hex0, as synthesized in air at 1400 °C followed by fast cooling to RT), 3.39 (Hex2, obtained by high-pressure oxygen annealing under 215 bar of pure oxygen at 400 °C followed by slow cooling of the as-made sample to RT) and 3.28 (Hex1, obtained by annealing in oxygen at 270 °C followed by fast cooling to RT of the high pressure oxygen loaded sample).
2.2 Material characterization
The structural X-ray studies of the synthesized oxides were carried out at room temperature (RT) in the range of 10–110 degrees with CuKα radiation using a PANalytical Empyrean diffractometer. For high-temperature measurements, an Anton Paar HTK 1200N oven-chamber was installed. Measurements in pure O2 using a flow rate of 100 cm3 min−1 were conducted for 15 min per scan. To observe the structural changes during the long-term oxidation at a carefully chosen temperature, the sample was rapidly heated (ca. 5 min) to 250 °C. Additional measurements were conducted for the temperature increases and decreases of 10 °C in a range of 220–300 °C for the HoMnO3+δ 15MM sample. The XRD tests of the phase stability and thermal expansion in air were also carried out up to 900 °C with the data taken every 100 °C. The GSAS/EXPGUI set of software was used for the Rietveld analysis of the data.34,35Scanning electron microscopy (SEM) evaluation of the chemical composition and the element mapping were done for powders using JEOL JSM-7610F apparatus equipped with an EDS detector by using a magnification range of 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
The OSCs during reduction/oxidation runs were determined by using the thermogravimetric (TG) method. All experiments were conducted on a TA Q5000 IR apparatus. For all the studies, either pure O2 or synthetic air flow at 100 cm3 min−1 and heating rates of 0.1–5° min−1 were used. Prior to the studies in an oxygen atmosphere, all the investigated samples were heated up to 500 °C and cooled down to RT with a heating rate of 5° min−1 in a flow of synthetic air to remove possible surface contamination. As shown in the following paragraphs, this annealing resulted in a slight oxygen adsorption and δ > 0 for some of the samples at the beginning of the TG experiments. The temperatures of reduction and oxidation, Tred and Toxi, were evaluated by determining the extremes of the derivative of the TG curve. Stability studies were also conducted in air atmosphere for 7 days at the temperatures where maximum oxygen content was observed as determined from the TG measurements. Based on previous reports,7,8 the oxygen content was fixed as equal to 3 (δ = 0) at 500 °C.
XAS measurements were performed at the 2A undulator beamline of PLS-II (Pohang Light Source-II) by employing the total electron yield (TEY) method. Samples were cleaned in situ in vacuum by repeated scraping with a diamond file under pressure higher than 2 × 10−10 Torr. XAS data were obtained at T ∼ 350 K in order to reduce the charging problem in these insulating oxides. The experimental energy resolution for the XAS data was set to ∼100 meV at hν ∼ 600 eV. All the XAS spectra were normalized to the incident photon flux. Magnetization measurements were performed using a Magnetic Property Measurement System (Quantum Design). Temperature dependence of magnetization was measured in a magnetic field H = 1 kOe in the temperature range of 2–395 K, initially in a “zero field cooling” mode (cooling the sample to T = 2 K in a zero magnetic field, switching a magnetic field on, and measuring upon warming), then in a “field cooling” mode (measuring upon cooling in a magnetic field) and finally the remanent magnetization was measured upon warming in the zero magnetic field after switching the magnetic field off at T = 2 K.
3. Results and discussion
3.1 Structure and the oxygen storage properties of HoMnO3+δ during temperature swing in oxygen
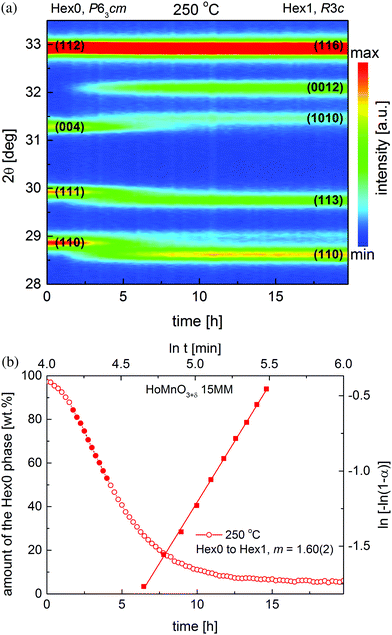
To observe the structural evolution caused by oxygen incorporation in situ, the oxygen stoichiometric, nearly single-phase HoMnO3 15MM (containing traces of Ho2O3) sample was rapidly, in about 5 min, heated from room temperature to 250 °C and held in pure oxygen for 20 hours. As can be seen from the time-dependent XRD data presented in Fig. 1a, the HoMnO3 15MM transforms completely from Hex0, the P63cm (δ ≈ 0) phase to the oxidized phase Hex1, R3c (δ ≈ 0.28) in a matter of 8–10 hours. The progression of structural transition is relatively slow, and therefore, easily observable during XRD measurements when the P63cm (Hex0) diffraction peaks gradually disappear and the R3c peaks (Hex1) emerge. During oxidation, oxygen is intercalated into the Mn–O sublattice, resulting in an increase in the average Mn coordination number from 5 in trigonal-bipyramids to an average of 8, while the 8-fold Ln–O coordination is preserved. However, some of the allowed 8 oxygen sites around the Mn ion are only partially occupied to satisfy the proper bond lengths. A fraction of the oxygen ions becomes shifted from their original positions, which together with the incorporation of additional oxygen results in a rearrangement of the stacking mode of the Mn–O layers, and the formation of the R3c superstructure with a tripling of the z-parameter along the c-axis, as documented in previous works.8,26 In particular, no peak shift is observed during the oxidation process, which confirms that the mechanism of the transition is a two phase-like, i.e., there are no regions in the material having an intermediate oxygen content.Refinement of the data presented in Fig. 1a allowed for the determination of changes in the phase content in the studied material, with disappearance of the Hex0 phase and formation of the Hex1 phase as presented in Fig. 1b (circles). The nature of this transition (rate-controlling process) was examined by using the approach presented by Motohashi et al.36 for the determination of the properties of the BaYMn2O5+δ system. Following the Avrami equation, a plot of ln(−ln(1 − α)) vs. ln(t) has been obtained, in which α is a time-dependent fraction of the reacted phase i.e., Hex0 (squares in Fig. 1b). The typical range of data, 0.15 < α < 0.5 (corresponding to the full circles), was used in calculations,37 as presented in Fig. 1b. The fitted value of the slope coefficient m was found to be equal to 1.6, suggesting, according to the Hancock and Sharp approach, a large influence on the surface reaction and new phase nucleation, both limiting the speed of the oxidation process.36,37
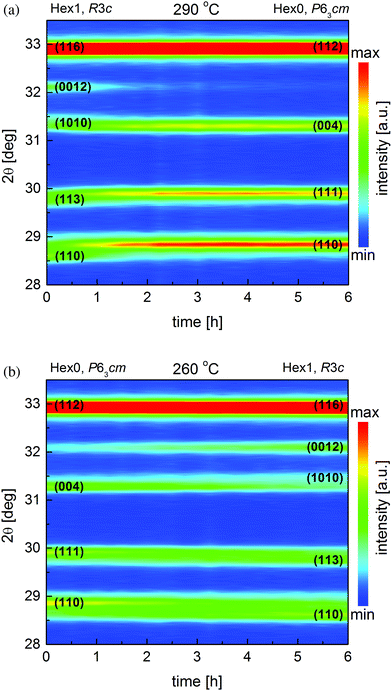
Additional detailed structural evaluation of the oxidized HoMnO3+δ 15MM sample was done in the temperature range of 220–300 °C. Starting from the fully oxidized Hex1 material at 250 °C, the temperature was increased by 10 °C each time, and then 24 consecutive isothermal scans, each lasting ∼15 min, were carried out for a total of 6 hours. No changes in the crystal structure of the Hex1 material, besides the thermal lattice expansion, were observed up to 270 °C. At 280 °C, the stoichiometric Hex0 phase begins to form, however, due to very slow kinetics at that temperature, the transition did not proceed to completion after 6 hours. Nevertheless, at 290 °C, a complete disappearance of the Hex1 peaks and emergence of the Hex0 peaks could be clearly observed (Fig. 2a). At 300 °C, the sample retained the Hex0 structure. The results presented here show that the phase stability diagram (in oxygen) of the various LnMnO3+δ oxides, as obtained by Abughayada et al.,8 during a faster heating procedure is not precise. In this particular case of HoMnO3+δ 15MM, the estimated temperature of the Hex1 → Hex0 phase transition on heating by long-time equilibration experiments is about 40 °C lower than that reported in ref. 8. This discrepancy can be explained by taking into account the influence of mechanical milling that lowers the particle size and breaks the agglomerates, on the properties of the material. As shown before for BaLnMnO5+δ Ln = Pr, Sm, milling significantly lowers the characteristic temperature of reduction.25 In addition, since the oxygen partial pressure (pO2) was kept constant during experiments (1 atm), from the thermodynamic point of view, there should be no regions in the equilibrium phase diagram with both phases thermodynamically stable. However, due to the limitations of slow kinetics (the new phase nucleation and growth, etc.), such mixed-phase regions are observed depending on the heating rate or holding time; for example, in the reported case here at 280 °C on heating, the equilibrium is not achieved during the 6 hour hold. However, it could be expected that at this temperature, the Hex0 phase would be stable after a very long equilibration time.
Structural studies of the Hex0 HoMnO3 15MM sample continued during the cooling down experiments from 300 °C to 220 °C. At 270 °C, traces of the oxidized Hex1 phase were already detected; however, during 6 hour time of the measurements, the transition was not finished. A decrease in temperature down to 260 °C caused further structural transformation of the material (Fig. 2b); however, the Hex0 phase completely disappeared only at 240 °C. This is in agreement with our initial observation of the Hex0 phase transformation to Hex1 at 250 °C, which took about 8–10 hours, so it was not completed here upon cooling at 250 °C during the 6 hour hold. The single-phase Hex1 was preserved down to 220 °C and no traces of the more oxidized Hex2 phase were detected. Considering very slow kinetics and the appearance of the Hex1 phase already at 270 °C upon cooling, the temperature hysteresis of the Hex0 ↔ Hex1 transition could possibly be estimated as only 10 °C (280 °C upon heating, 270 °C upon cooling). A small value of the hysteresis temperature of transitions is an important parameter for conceivable application of OSM utilizing temperature swing processes.8
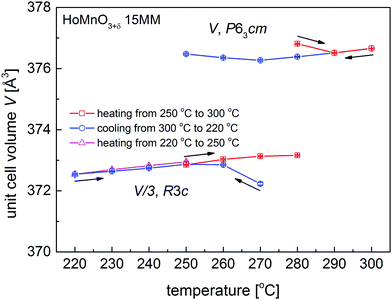
Calculated unit cell volumes V of the Hex0 and Hex1 phases during heating (250–300 °C) and cooling (300–220 °C), and during additional heating (220–250 °C) are presented in Fig. 3 (unit cell parameters a and c are shown in Fig. S1a and b, ESI†). The c and V parameters were normalized for the R3c phase by dividing by 3. Interestingly, for the newly appearing phases (i.e. Hex0 at 280 °C upon heating and Hex1 at 270 °C upon cooling), the calculated parameters diverge from the overall trend, which can be associated with the internal strain, evolution of the oxygen content in forming phases or resulting from the two-phase refinement of poorly formed phases. Deviation from the general trend is much more visible for the parameter a, because the additional oxygen is preferentially incorporated into or removed from the Mn–O planes. The final XRD data created by adding up all the 24 measurements at respective temperatures for the single-phase phases Hex0 at 300 °C and Hex1 at 220 °C are presented in Fig. 4, together with the Rietveld refinements using the P63cm and R3c space groups, respectively. The structural parameters are listed in Table 1.
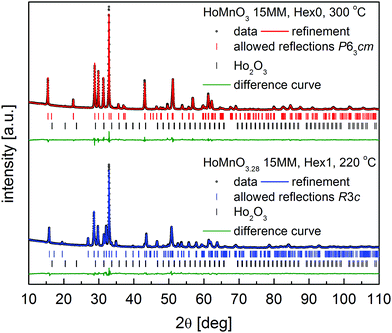 | ||
| Fig. 4 Diffractograms with Rietveld analysis for: HoMnO3 15MM at 300 °C (Hex0), and HoMnO3.28 15MM at 220 °C (Hex1); see details in the text. | ||
| Phase composition | T [°C] | Space group | a [Å] | c [Å] | V [Å3] | R wp [%] |
|---|---|---|---|---|---|---|
| Hex0, δ = 0 | 300 | P63cm | 6.1769(1) | 11.3993(2) | 376.66(1) | 2.27 |
| Hex1, δ = 0.28 | 220 | R3c | 6.2181(1) | 33.3777(9) | 1117.63(6) | 2.32 |
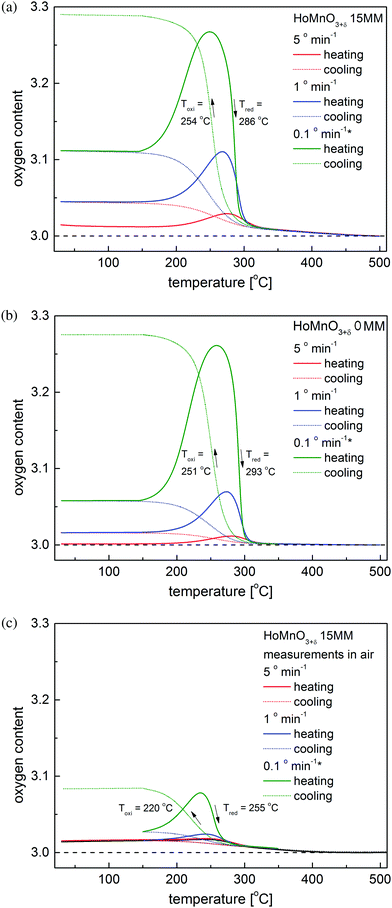
Changes in the oxygen stoichiometry with temperature for the HoMnO3+δ 15MM sample were investigated using the thermogravimetric technique and compared with the structural data. The results of the temperature swing processes carried out consecutively at different heating rates (5° min−1, 1° min−1, and then 0.1° min−1) are presented in Fig. 5a. For comparison, the data for the initial HoMnO3+δ 0MM sample are shown in Fig. 5b. Based on these measurements, the oxygen storage-related properties of the studied samples have been derived and are presented in Table 2. While comparing the oxygen incorporation into the as-obtained material and the ball-milled sample, some increase in the speed of the processes can be observed as a higher oxygen content at the respective maxima for the same heating/cooling speeds and a small decrease in the temperature hysteresis of the Hex0 ↔ Hex1 transition ΔT = Tred − Toxi can be observed for the 15MM sample. This is consistent with the SEM results (Fig. S2 and S3, ESI†), showing no significant effect of mechanical milling process on the morphology of the powders. Furthermore, EDX elemental mapping showed the expected, homogeneous distribution of the elements in the initial, as well as in the milled samples.
| Chemical composition | Atmosphere | OSC between RT and 500 °C [wt%] | Maximum oxygen content during 0.1° min−1 heating | Temperature of the maximum oxygen content on 0.1° min−1 heating [°C] | ΔT [°C] |
|---|---|---|---|---|---|
| HoMnO3+δ 0MM | Oxygen | 1.64 | 3.26 | 260 | 42 |
| HoMnO3+δ 15MM | Oxygen | 1.73 | 3.27 | 250 | 32 |
| HoMnO3+δ 15MM | Air | 0.50 | 3.08 | 235 | 35 |
3.2 Occurrence of the oxidized HoMnO3.28 15MM Hex1 phase in air
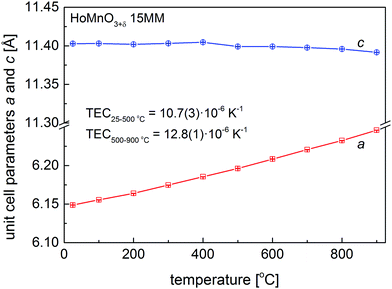
As shown above, the temperature swing process progresses effectively for HoMnO3+δ 0MM and HoMnO3+δ 15MM in an atmosphere of pure oxygen. It was therefore of interest to investigate if the oxidized Hex1 phase would also appear in air at elevated temperatures. Initial high-temperature XRD measurements have shown no presence of the Hex1 phase for the HoMnO3 15MM sample in air up to 900 °C. Since the oxygen stoichiometry was preserved, these measurements allowed for the calculation of the Hex0 unit cell parameters and the determination of the thermal expansion coefficient (TEC), as depicted in Fig. 6. The thermal expansion was found to be highly anisotropic, with almost no change in the c parameter up to 400 °C and a slight decrease at higher temperatures indicating persistence of filling the e′2 and e′′2 electronic orbitals of MnO5. The unit cell volume-based values were refined as TEC25–500°C = 10.7(3) × 10−6 K−1 and TEC500–900°C = 12.8(1) × 10−6 K−1 for the Hex0 phase, which are comparable with Dy-rich Dy1−xYxMnO3.7Additionally, more accurate diffraction measurements were performed every 10 °C in the 200–300 °C temperature range (Fig. S4, ESI†), clearly indicating an appearance of a low-intensity extra reflection at 220–260 °C, which was identified as the (0012) diffraction peak of the oxidized Hex1 phase. By considering the slow kinetics of the Hex0 ↔ Hex1 transitions observed in the oxygen (Fig. 5a and b), the additional long-term, 7 day annealing tests were conducted for the HoMnO3+δ 15MM sample in air at 250 °C. As shown in Fig. 7, the Rietveld analysis indicated the two-phase mixture of about 70 wt% of the oxidized Hex1 and 30 wt% of Hex0 phases, which would indicate the total oxygen content of about 3.20. Thermogravimetric measurements (Fig. 5c) confirmed the partial oxidation of the material in air; however, the amount of incorporated oxygen, even during slow cooling (0.1° min−1), was relatively small, δ ≈ 0.08. Remarkably, the Hex1 to Hex0 transition temperature upon heating Tred ≈ 255 °C was found to be significantly lower than that in the oxygen, ca. 290 °C, implying its strong dependence on the oxygen pressure. The excess oxygen achieved in air during a TG experiment is lower than that observed for DyMnO3+δ (δ ≈ 0.22) (see Fig. 3 in ref. 7), but confirms the temperature swing process conducted in air to be successful for smaller size lanthanide hexagonal manganite HoMnO3+δ that can be easily prepared in air. It seems thus that the proper selection of lanthanide could open a possibility for the usage of hexagonal manganites as OSMs in air at controllable temperatures in the range of 200–300 °C, where the waste heat is frequently available in many industrial processes.
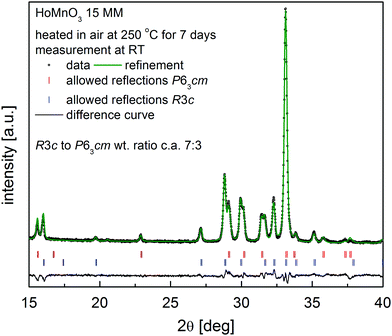 | ||
| Fig. 7 XRD pattern together with Rietveld analysis for the HoMnO3+δ 15MM sample after heating at 250 °C in air for 7 days, showing the formation of ∼70 wt% of the oxidized Hex1 phase. | ||
3.3 Valence states of Mn and Ho ions in HoMnO3+δ 0MM determined from XAS
Fig. 8a shows the Mn 2p (L-edge) XAS spectra of HoMnO3+δ 0MM (δ = 0, 0.28, 0.4), measured at T = 350 K. As a guide to the valence states of Mn ions in HoMnO3+δ 0MM, they are compared with those of reference Mn oxides of formally divalent (Mn2+) MnO, formally trivalent (Mn3+) Mn2O3, and formally tetravalent (Mn4+) MnO2.38,39 L3 (2p3/2) and L2 (2p1/2) peaks arise from the spin–orbit splitting of the Mn 2p core levels. The weakly negative slope for the δ = 0 sample (Hex0) is due to charging in the Hex0 sample. It is well known that the Mn 2p XAS peak positions shift toward higher energies as the Mn valence increases.36,38 This figure shows clearly that the peak positions of the Mn 2p XAS spectra of HoMnO3+δ 0MM shift as δ changes, with the accompanying changes in their line shapes. The Mn 2p XAS spectrum of δ = 0 (Hex0) is very similar to that of Mn2O3, while that of δ = 0.4 (Hex2) is similar to that of MnO2 and that of δ = 0.28 (Hex1) is in between those two. These differences provide evidence that the valence states of Mn ions, ν(Mn), increase monotonically from δ = 0 to δ = 0.4. In other words, ν(Mn) ≈ 3+ at δ = 0 to ν(Mn) ≈ 4+ at δ = 0.4. These Mn 2p XAS results support the oxygen content determined by the TG method (see Fig. 5 and Table 2).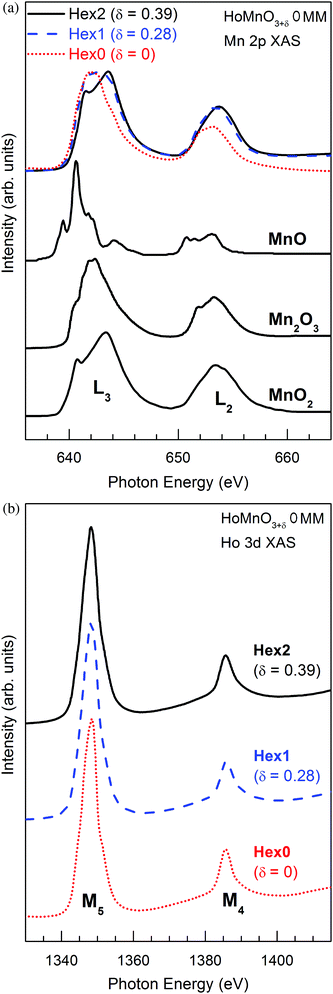 | ||
| Fig. 8 XAS spectra of HoMnO3+δ 0MM (δ = 0, 0.28, 0.4) measured at T = 350 K for (a) the Mn 2p (L-edge) and (b) the Ho 3d (L-edge). | ||
In contrast, as shown in Fig. 8b, the Ho 3d (L-edge) XAS spectra of HoMnO3+δ 0MM (δ = 0, 0.28, 0.4) do not change for the varying δ. This observation indicates that the valence states of Ho ions do not change with δ, keeping the trivalent states (Ho3+) for all δ. Our XAS study for Mn 2p and Ho 3d states suggests that the excess oxygen in HoMnO3+δ 0MM causes the electron transfer from Mn3+ to O (oxygen), resulting in Mn4+ at δ = 0.4 (Hex2) and Mn3+–Mn4+ mixed-valent at δ = 0.28 (Hex1), but the excess oxygen does not affect Ho ions in HoMnO3+δ 0MM.
3.4 Magnetic measurements of oxygen loaded HoMnO3+δ 0MM
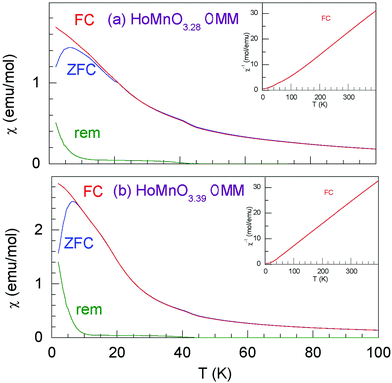
Temperature dependences of the molar magnetic susceptibility for HoMnO3.28 0MM and HoMnO3.39 0MM are presented in Fig. 9. A cusp in the “zero field cooled” magnetization is observed at T = 6.5 K for both compositions. The “zero field cooled” and “field cooled” magnetizations show irreversibility at low temperatures. The above features together with an observation of the remanent magnetization point at possible spin glass behavior in both materials. The inverse molar susceptibility presented in the insets for the “field cooled” measurements demonstrate a linear behavior above T ∼ 150 K. We fitted our susceptibility data to a modified Curie–Weiss formula χ = χ0 + (1/8) × μeff2 × (T − TC), where χ is the molar susceptibility, χ0 is the background susceptibility, μeff = gS(S + 1) is the paramagnetic effective moment, S is the magnetic spin, and g = 2 is the Lande factor. The fitting results for the “field cooled” data in the temperature range of 200–390 K give the values of μeff approximately equal to 9.2 μB (Bohr magnetons) and 9.25 μB for HoMnO3.28 0MM and HoMnO3.39 0MM, respectively. The susceptibility is dominated by the magnetic contribution of Ho3+ moments (μHoeff = 10.6 μB). An addition of Mn3+ (μMneff = 4.9 μB) or Mn4+ (μMneff = 3.87 μB) both present in HoMnO3+δ 0MM materials would give the total effective moment according to the simple formula (μeff)2 = (μHoeff)2 + (μMneff)2, which gives μeff = 11.68 μB for Mn3+ and μeff = 11.28 μB for Mn4+ that are close to each other, and a small change in the Mn valence results in a hardly detectable change in μeff. Our values of μeff are significantly smaller than those expected from the above-simplified model. The same discrepancy was observed for Dy1−xYxMnO3+δ materials.264. Conclusions
Hexagonal HoMnO3+δ manganites prepared by various methods exhibit interesting properties related to their application in the thermal swing process of oxygen incorporation and release. The long-term annealing experiments have shown that a Hex0 → Hex1 transition for HoMnO3+δ 15MM is limited by the surface reaction and nucleation of the new phase. The temperatures of reversible Hex0 ↔ Hex1 structural transitions caused by a change in oxygen stoichiometry between δ = 0 and 0.28 have been established as 290 °C on heating and 250 °C on cooling, also indicating that temperature hysteresis of the transition could possibly be as small as 10 °C. High impact ball-milling for 15 min had only a small effect on improving the speed of the reduction/oxidation processes. Interestingly, the Hex1 phase for the HoMnO3+δ 15MM material can also be obtained by prolonged heating in air at a temperature range of 220–255 °C, which is important for practical applications of the thermal swing process.X-ray absorption spectroscopy measurements confirmed that oxygen loading of the HoMnO3+δ 0MM material causes the electron transfer from Mn3+ to oxygen, resulting in Mn4+ at δ = 0.39 (Hex2) and Mn3+–Mn4+ mixed-valent at δ = 0.28 (Hex1), but that the excess oxygen does not affect Ho ions in HoMnO3+δ 0MM. An attempt to resolve the effective Mn valence from magnetic susceptibility data gave inconclusive results due to dominating magnetism of Ho3+ ions.
Comparison of hexagonal HoMnO3+δ with DyMnO3+δ and substituted Dy1−xYxMnO3+δ materials indicates that a tiny increase in the ionic size of the lanthanide has a huge effect on the redox properties of hexagonal manganites. As such, an increased effort should be directed at synthesizing and studying the larger average size (Y,Ln)MnO3+δ compounds.
Acknowledgements
This project was funded by the National Science Centre, Poland, on the basis of the decision number UMO-2015/19/B/ST8/00871. The work at the CUK was supported by the Korean National Research Foundation (NRF) under the contract number (No. 2016R1D1A1B03932391). A synchrotron radiation spectroscopy experiment was supported by the PAL (Pohang Accelerator Laboratory) and MSIP in Korea.References
- J. Emsley, Nature’s Building Blocks: An A–Z Guide to the Elements, Oxford University Press, 2001 Search PubMed.
- M. Kanoglu, I. Dincer and M. A. Rosen, Int. J. Energy Res., 2008, 32, 35–43 CrossRef CAS.
- D. R. Vinson, Comput. Chem. Eng., 2006, 30, 1436–1446 CrossRef CAS.
- Y.-S. Lin, D. Mac Lean and Y. Zeng, High temperature adsorption process, US Pat., 6059858, 2000 Search PubMed.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS.
- S. S. Hashim, A. R. Mohamed and S. Bhatia, Renewable Sustainable Energy Rev., 2011, 15, 1284–1293 CrossRef CAS.
- S. Remsen and B. Dabrowski, Chem. Mater., 2011, 23, 3818–3827 CrossRef CAS.
- C. Abughayada, B. Dabrowski, S. Kolesnik, D. E. Brown and O. Chmaissem, Chem. Mater., 2015, 27(18), 6259–6267 CrossRef CAS.
- A. Klimkowicz, K. Świerczek, A. Takasaki, J. Molenda and B. Dabrowski, Mater. Res. Bull., 2015, 65, 116–122 CrossRef CAS.
- T. Motohashi, T. Ueda, Y. Masubuchi, M. Takiguchi, T. Setoyama, K. Oshima and S. Kikkawa, Chem. Mater., 2010, 22, 3192–3196 CrossRef CAS.
- K. Świerczek, A. Klimkowicz, K. Zheng and B. Dabrowski, J. Solid State Chem., 2013, 203, 68–73 CrossRef.
- T. Kodama and N. Gokon, Chem. Rev., 2007, 107, 4048–4077 CrossRef CAS PubMed.
- J. Kašpar and P. Fornasiero, J. Solid State Chem., 2003, 171, 19–29 CrossRef.
- Z. Yang, Y. S. Lin and Y. Zeng, Ind. Eng. Chem. Res., 2002, 41, 2775–2784 CrossRef CAS.
- Y. Wei, H. Wang, F. He, X. Ao and C. Zhang, J. Nat. Gas Chem., 2007, 16, 6–11 CrossRef CAS.
- K. Li, H. Wang and Y. Wei, J. Chem., 2013, 294817 Search PubMed.
- K. Wang, Q. Yu and Q. Qin, J. Therm. Anal. Calorim., 2013, 112, 747–753 CrossRef CAS.
- S. Bhavsas and G. Veser, RSC Adv., 2014, 4, 47254–47267 RSC.
- O. L. Pineda, Z. L. Moreno, P. Roussel, K. Świerczek and G. H. Gauthier, Solid State Ionics, 2016, 288, 61–67 CrossRef CAS.
- J. Vieten, B. Bulfin, F. Call, M. Lange, M. Schmücker, A. Francke, M. Roeba and C. Sattler, J. Mater. Chem. A, 2016, 4, 13652–13659 CAS.
- T. Motohashi, M. Kimura, Y. Masubuchi, S. Kikkawa, J. George and R. Dronskowski, Chem. Mater., 2016, 28(12), 4409–4414 CrossRef CAS.
- M. Gilleßen, M. Lumeij, J. George, R. Stoffel, T. Motohashi, S. Kikkawa and R. Dronskowski, Chem. Mater., 2012, 24, 1910–1916 CrossRef.
- A. Klimkowicz, K. Świerczek, K. Zheng, M. Baranowska, A. Takasaki and B. Dabrowski, Solid State Ionics, 2014, 262, 659–663 CrossRef CAS.
- T. Motohashi, T. Ueda, Y. Masubuchi and S. Kikkawa, J. Ceram. Soc. Jpn., 2011, 119, 894–897 CrossRef CAS.
- A. Klimkowicz, K. Świerczek, T. Yamazaki and A. Takasaki, Solid State Ionics, 2016, 298, 66–72 CrossRef CAS.
- C. Abughayada, B. Dabrowski, S. Remsen, S. Kolesnik and O. Chmaissem, J. Solid State Chem., 2014, 217, 127–135 CrossRef CAS.
- A. Smith, A. Sleight and M. Subramanian, Mater. Res. Bull., 2011, 46, 1–5 CrossRef CAS.
- S. Ishiwata, Y. Tokunaga, Y. Taguchi and Y. Tokura, J. Am. Chem. Soc., 2011, 133, 13818–13820 CrossRef CAS PubMed.
- K. Uusi-Esko and M. Karppinen, Chem. Mater., 2001, 23, 1835–1840 CrossRef.
- H. W. Brinks, H. Fjellvåg and A. Kjekshus, J. Solid State Chem., 1997, 129, 334–340 CrossRef CAS.
- S. Remsen, B. Dabrowski, O. Chmaissem, J. Mais and A. Szewczyk, J. Solid State Chem., 2011, 184, 2306–2314 CrossRef CAS.
- F. M. F. de Groot, J. C. Fuggle, B. T. Thole and G. A. Sawatzky, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 42, 5459–5468 CrossRef CAS.
- G. van der Laan and I. W. Kirkman, J. Phys.: Condens. Matter, 1992, 4, 4189–4204 CrossRef CAS.
- A. C. Larson and R. B. von Dreele, Los Alamos Natl. Lab. Rep. – LAUR, 2004, 86–748 Search PubMed.
- H. B. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef.
- T. Motohashi, T. Ueda, Y. Masubuchi and S. Kikkawa, J. Phys. Chem. C, 2013, 117, 12560–12566 CAS.
- J. D. Hancock and J. H. Sharp, J. Am. Ceram. Soc., 1972, 55, 74–77 CrossRef CAS.
- C. Mitra, Z. Hu, P. Raychaudhuri, S. Wirth, S. I. Csiszar, H. H. Hsieh, H.-J. Lin, C. T. Chen and L. H. Tjeng, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 92404 CrossRef.
- P. Ghigna, A. Campana, A. Lascialfari, A. Caneschi, D. Gatteschi, A. Tagliaferri and F. Borgatti, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 132413 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cp03556j |
| This journal is © the Owner Societies 2017 |