Coherent intermolecular proton transfer in the acid–base reaction of excited state pyranine†
Abstract
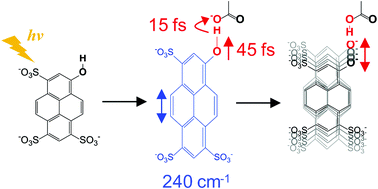
Detailed molecular dynamics simulations of an acid–base reaction have been the subject of extensive investigations. Here we report the excited state proton transfer dynamics of pyranine (8-hydroxypyrene-1,3,6-trisulfonic acid, HPTS) in acetate buffer by time-resolved fluorescence (TF) and quantum mechanical/effective fragment potential molecular dynamics (QM/EFP-MD) simulations. High time resolution in TF and TF spectra measurements allows the acquisition of accurate reaction kinetics. Upon the photoexcitation of HPTS, the proton (deuterium) is transferred coherently to acetate in 60 fs (80 fs) for a contact pair of HPTS (DPTS) and acetate by a hydrogen bond, which comprises approximately 28% of the population. ESPT proceeds slowly on a picosecond time scale for the remaining HPTS as reported previously. Coherent wave packet motions of the reactant (acid) and the product (conjugate base) enable the acquisition of the vibrational spectra of excited states via TF (VETF). A comparison of the VETFs of the reactant and the product and the calculation of the Huang–Rhys factors (vibrational reorganization energies) identify the vibrational modes that actively participate in the coherent proton transfer. In particular, the 246 cm−1 vibrational mode, which consists of in-plane skeletal stretching motion, promotes the ESPT by transferring the donor oxygen towards the acceptor oxygen in acetate. QM/EFP MD simulations corroborate the experiment and provide molecular details of the ESPT.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...