Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective crystallisation of carbamazepine polymorphs on surfaces with differing properties†
Huaiyu
Yang
 ,
Cai L.
Song
,
Ying X. S.
Lim
,
Wenqian
Chen
,
Cai L.
Song
,
Ying X. S.
Lim
,
Wenqian
Chen
 and
Jerry Y. Y.
Heng
and
Jerry Y. Y.
Heng
 *
*
Department of Chemical Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, UK. E-mail: jerry.heng@imperial.ac.uk
First published on 14th September 2017
Abstract
Surface-induced nucleation of carbamazepine (CBZ) in ethanol was investigated with different surface materials: glass, polytetrafluoroethylene (PTFE) and tin. The introduction of foreign surfaces with different areas and surface chemistries into the solution has an impact on the crystal morphology and polymorphic form (Form II or III). With an increase in supersaturation, a higher possibility of crystallisation of CBZ metastable Form II was observed, as expected. Increasing the number of inserts resulted in a direct increase in the surface area available for heterogeneous nucleation. The increase in surface area resulted in the greater possibility of obtaining the metastable Form II of CBZ. The stable Form III preferred to nucleate on tin rather than on glass and PTFE. The results indicate that the two different polymorphs of CBZ can be selectively crystallised out from solution with the aid of a foreign surface. The kinetic mechanism of heterogeneous nucleation of the different polymorphs induced by foreign surfaces was discussed. The potential applications will be used to control and design the crystallisation process.
Introduction
Crystallisation is widely used as a purification technique for active pharmaceutical ingredients (APIs) in the pharmaceutical industry.1 Different polymorphs of APIs may appear in a crystallisation process. With different arrangements in the crystal lattice, polymorphs have different physicochemical properties such as solubility and melting point.2,3 These differences directly affect the performance properties of the APIs such as shelf life, efficacy and bioavailability.4 Polymorphic purity is essential for quality control, as the presence of any undesirable polymorphs or polymorphic transformation during manufacturing processes induces substantial economic loss (for example, more than $250 million loss due to an unknown more stable form of ritonavir, an antiviral compound for AIDS).5 The evaporation crystallisation processes of butyl paraben in liquid–liquid phase separation are very different on glass, metal and PTFE surfaces,6,7 and ibuprofen changes morphology on Al, Au or self-assembled –CH3, –OH, and –COOH monolayers.8 On a siloxane monolayer template, a metastable polymorph of 1,3-bis(m-nitrophenyl)urea appeared.9 Porosity10 and roughness11 of surfaces also affect the crystallization of proteins,11–14 glass15 and colloids.16 It is important to understand the effect of surface properties on the crystallisation from a process development perspective to optimise the surfaces of crystallisers and to achieve better control of the crystallisation process.Carbamazepine (CBZ) is commonly used as an anticonvulsant and pain-relieving drug. Five anhydrous polymorphs of CBZ were reported previously17 and only four forms (i.e. I, II, III & IV) are commonly observed.18 Form III is thermodynamically stable between ambient temperature and 70 °C,19 while Forms I, II and IV are metastable but sometimes kinetically favoured.20 It was reported previously that the degree of supersaturation and the solvents determine the polymorph outcome of CBZ.2,21 In this work, we focus on the effect of introducing new foreign surfaces with differing properties, i.e. different areas of the surfaces and different materials of the surfaces, on the polymorph of CBZ. The study was conducted at two different degrees of supersaturation in cooling crystallisation in ethanol. The CBZ crystal structure, the interactions between surfaces and CBZ molecules, and the pre-exponential factors in nucleation are discussed.
Experimental
Materials
Carbamazepine (5H-dibenzazepine-5-carboxamide) was purchased from Sigma Aldrich and AnalaR NORMAPUR® ethanol was purchased from VWR. Both chemicals were used as-received. Micro-haematocrit capillary tubes from Jaytec Glass Ltd (1.55 mm outer diameter), tin-coated wires (1.21 mm outer diameter) and PTFE tubing (1.52 mm outer diameter) were used. 40 mm of each material was placed in a 7 ml glass vial (moulded pathology media vials, Neutral Type 1B glass, VWR International).Crystallisation of carbamazepine
Solutions (3 ml) with CBZ concentrations of 40.0, 50.0, 60.0, 65.0 and 70.0 mg ml−1 in ethanol were prepared in glass vials. Glass capillaries, PTFE tubes and tin wires were cut to 40 mm length and submerged (∼15 mm) in the CBZ/ethanol solutions. The surface area of each vial in contact with the solution was ∼950 mm2 (initial surface area), adding extra 5.9–7.7% contact area (foreign surface area) for each insert (capillary/tube/wire) submerged in the solution. 2 or 8 inserts with random configurations were placed in the vials with the solution. The vials were sealed with caps and Parafilm on the outside to prevent evaporation of the solvent. The solutions were heated up to 70 ± 1 °C for about one hour to ensure all the solid CBZ dissolved and to minimise any history effect.22 Then, the solutions were cooled down to 20 °C in 30 minutes inside a water bath controlled by a circulator (Grant LT). The crystallisation process was recorded using a USB camera (endoscope). For each condition, at least six experiments were repeated; the shapes of all the crystals in the solution were observed at about 3.5 hours after the solution temperature reached 20 °C. The number of experiments with the appearance of only needle crystals, prismatic crystals or both needle and prismatic crystals in the whole solution (both on the bottom of the vials and on the foreign surfaces) was compared with the total number of parallel experiments performed, and the percentage of occurrence of Form II (only needle crystals observed), Form III (only prismatic crystals observed) and both Forms II and III (both needle and prismatic crystals observed) was determined.Results and discussion
Only two different morphologies (prismatic and needle-shaped crystals, i.e. Forms III and II, respectively) were observed in this work. The CBZ crystals of needle and prismatic shapes were Forms II and III, respectively, as confirmed by XRPD (Fig. 1). As Form III is a stable polymorph, there is no transition from Form III to Form II, which is consistent with the observation in experiments. Form III seeds were added to the ethanol solution, but no Form II crystals were formed under the range of experimental conditions in this work. It is reported that Form II will transform to Form III under stirring conditions,23,24 but in this work, the needle-shaped crystals did not change shape after 5 weeks, indicating no obvious transition from Form II to Form III without stirring. At 20 °C, the solubility of Form III in ethanol is 18.60 mg ml−1 and the difference in solubility between Form II and Form III at the temperature range T = 20–60 °C is almost constant.13 The solubility of Form II is 1.1 times (average) higher than the solubility of Form III in the temperature range between 10 °C and 30 °C.13 The supersaturation in this work is calculated based on the solubility of Form III.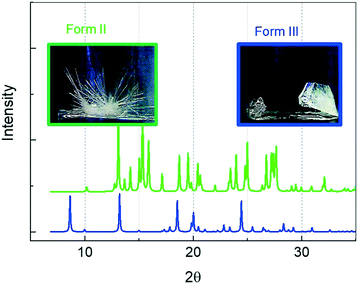 | ||
| Fig. 1 XRPD patterns for needle-shaped crystals (Form II with green colour) and prismatic shaped crystals (Form III with blue colour) of CBZ. | ||
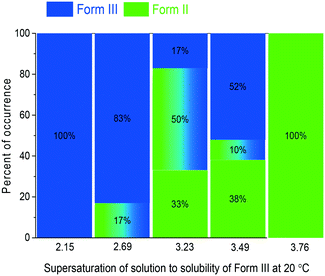
When the supersaturation was 2.15, no Form II crystals were obtained in the experiments. With the increase in supersaturation (S), the possibility of Form II formation increases from <7% at S = 2.69 to 100% at S = 3.76, as shown in Fig. 2. The supersaturation determines the polymorphs in the nucleation of CBZ, as higher supersaturation usually leads to metastable polymorphs, which is consistent with Ostwald's rule and as observed for several other systems. For L-histidine,25 eflucimibe,26L-glutamic acid,27 carbamazepine,2,28m-hydroxybenzoic acid and o-aminobenzoic acid,29 the metastable polymorph nucleated at high supersaturation, and the stable polymorph at low supersaturation.
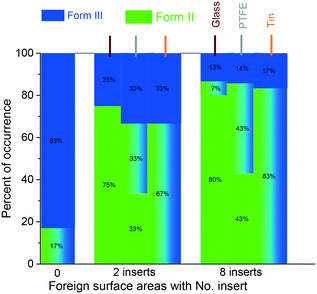
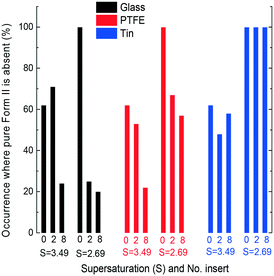
When foreign surfaces were introduced to the solutions, the percentage of polymorph outcome dramatically changed. Fig. 3 shows one case where Form II of CBZ nucleated and grew on the surface of glass capillaries and PTFE tubes, whereas Form III nucleated and grew on the surface of tin wire. In Fig. 3(a) and (b), the crystals on the bottom were Form II, the same as the crystals on the foreign surfaces, but the crystals in the solution in Fig. 3(c) were Form III. The foreign surface influences the nucleation at the solid–liquid interface, but may also influence the nucleation process in the bulk solution. In all the experiments shown in Fig. 4–7, at least several crystals were observed on both the foreign surface and the bottom of the vials. Therefore, there are three possibilities: 1 and 2: only prismatic or needle-shaped crystals on both foreign surfaces and the bottom of the vial, respectively, and 3: at least several prismatic and needle-shaped crystals on foreign surfaces or the bottom of the vial.
Fig. 4 shows that when 2 inserts of glass, PTFE or tin were brought into contact with the solution at S = 2.69 (Form III), the percentage of obtaining only Form III crystals dramatically decreased to <33% for all three materials. If 8 inserts (4 times larger foreign surface area) were in contact with the solution, the possibility of crystallising only Form III decreased to <17% for all three surfaces. With the same increase in area of the foreign surfaces, the possibility of obtaining only Form III increased in the order of materials of glass < PTFE < tin. The surface energy of glass,30 PTFE,31 and tin32 is 310, 19, and 709 mJ m−2, respectively.
It is noted that the trends in the polymorph (Form III only) occurrence for these three surfaces are opposite between the low supersaturation (S = 2.69) and the high supersaturation (S = 3.49). However, a direct link between the surface properties of these inserts and the polymorphic outcome is unclear. There is a competition between kinetics and thermodynamics33 on the polymorphic outcome, as also shown in our previous work on template-induced and solvent-induced polymorphic occurrence domains (TiPOD/SinPOD).12,13,34 The polymorphic outcome is affected by crystallisation conditions including concentration, the supersaturation range, temperature, solvent, the presence of additives, nucleating surfaces and several other external factors.35,36 In this work, glass and PTFE surfaces, which have lower surface energies than tin in this work, induced a high percentage of occurrence of Form II, which is consistent with the literature that the cyano surface induces more Form II than mercapto and fluoro surfaces, which have more than two times higher surface energy than the cyano surface.13 It is noticed that when 2 or 8 inserts of tin were added, if both Form II and Form III crystals were observed in the solution, nearly all the crystals on the tin surfaces would be Form III in the experiments, which indicates that, unlike glass and PTFE surfaces, tin surfaces prefer to generate Form III carbamazepine.
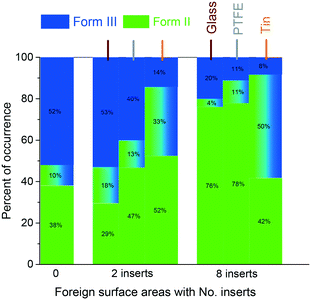
Fig. 5 shows that when the supersaturation was 3.49, introducing 2 inserts of foreign surfaces reduced the percentage of obtaining only Form III for the PTFE and tin surfaces, but a similar percentage for the glass surface. With 8 inserts in contact with the solution, the possibility of obtaining only Form III obviously decreased for all three surfaces. With tin surfaces in the solution, it was observed that Form III crystallised on the tin surfaces while Form II crystallised on the bottom in most of the experiments when Form II and Form III were observed simultaneously in one experiment. This is consistent with the experimental results at low supersaturation (Fig. 4) that the tin surface attracts Form III crystals.
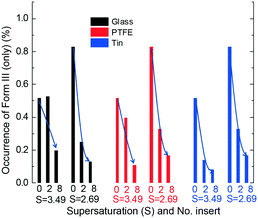
Fig. 6 shows that an increase in the number of foreign surfaces leads to a decrease in the percentage of Form III appearing (without any appearance of Form II) with all three material surfaces at two supersaturations. The foreign surfaces have a larger influence, i.e. a larger decrease of the polymorph occurrence percentage with an increase in area, for all cases except the glass inserts at higher supersaturation. The variation of obtaining only Form III with 2 inserts is much higher than the variation with 8 inserts (shown in the ESI†). The larger foreign surface area results in smaller differences between two supersaturations or different materials.
Fig. 4 and 5 show that at the same supersaturation, the influence of changing the surface properties (glass, PTFE and tin with more than 3 times differences) on polymorph appearance was greater than that of adding foreign surface areas (2 and 8 inserts with 2–3 times differences). Fig. 6 shows that when similar foreign surface areas with the same surface properties were introduced in the solution, the influence of higher supersaturation (less than 2 times difference) was greater than the influence of lower supersaturation (less than 1.5 times difference). The overall influence of the supersaturation ratio (2.69 and 3.49) was smaller than the influence of adding foreign surface areas (2 and 8 inserts). Based on the calculation from eqn (1), we can estimate the differences among polymorph occurrences at each supersaturation (S), surface area (A) or surface property (P) (shown in the ESI†). The influences of these three parameters are in the order: P (50% difference) > A (35% difference) > S (18% difference) in the range of this experimental work.
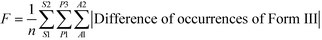 | (1) |
Fig. 7 shows the percentage of occurrence of at least one Form III crystal in the solution, which equals to the sum of the percentages of occurrence of only Form III crystals (shown in Fig. 6) and the percentage of occurrence of both Form II and Form III in the solution. Fig. 7 shows that the increase in the area of foreign surfaces leads to a decrease in the percentage of Form III outcome for the glass and PTFE surfaces. However, for the tin surfaces, there is no obvious trend at both low and high supersaturations with the increase in surface area. It is noticed that for most of the cases, in which Form II and Form III appear in one experiment, the crystals on the tin surface are Form III and the Form II crystals are located on the bottom of the vials. The results indicate that tin prefers to capture/hook polymorph Form III rather than Form II. This is consistent with the experimental results that if PTFE and tin inserts were introduced in the solution at the same time, Form III crystals preferably nucleated and grew on the tin surfaces, while Form II crystals always appeared on the PTFE surfaces, as shown in Fig. 8.
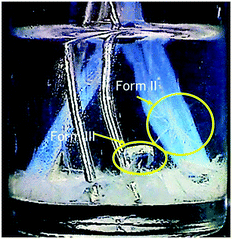 | ||
| Fig. 8 Both PTFE and tin surfaces were inserted into a carbamazepine–ethanol solution at S = 3.49. Form II crystallised on the PTFE surface whilst Form III crystallised on the tin surface. | ||
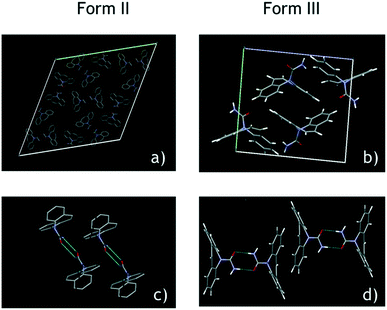
Form II has a crystal unit cell volume of 5.72 nm3,37 whereas Form III has a relatively very small cell volume of 1.17 nm3,38 which is more than 4 times smaller than that of Form II. The H-bond distance, –O⋯NH, calculated with Mercury software is 2.890 Å and 2.929 Å for Forms II and III, respectively, indicating a stronger H-bond in Form II than in Form III. Two typical groups of Form II and Form III CBZ molecules in a unit cell are shown in Fig. 9; the two groups of Form II stand parallel shoulder to shoulder, and the two groups of Form III are connected in a head-to-tail manner where the two close molecules look like they are embedded to each other. The crystal unit cells in Fig. 9 show that the H-bonds in Form III have only two directions which are nearly vertical to each other, and the H-bonds in Form III have eight different directions. If some CBZ molecules are attracted to the foreign surface, the stronger H-bonds of Form II and the higher possibility to attract other molecules from several directions may help to capture other CBZ molecules. The molecule in Form III has only one or two direction options to capture other CBZ molecules. The influence of the weaker H-bond between two CBZ molecules and the limited H-bond directions may make it more difficult to crystallise than Form II in heterogeneous nucleation on the foreign surfaces. CBZ molecules should have stronger interactions with glass (–OH) and PTFE (–F) surfaces than with tin surfaces, due to the weak H-bond between CBZ and –OH or –F, which may be the reason why Form II is favourable on glass and PTFE than on tin. This is consistent with the literature that the stronger H-bonds between CBZ and other molecules (solvents) hinder the stabilization of the interactions in the self-association of Form III before nucleation.21
Many experiments have shown that heterogeneous nucleation is more likely to occur at higher supersaturation, while homogeneous nucleation tends to occur at lower supersaturation.2,12,13,25,28 According to the classical nucleation theory (eqn (2)), this decrease in free energy results in a higher nucleation rate:
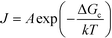 | (2) |
1. Form II and Form III are both nucleated by homogeneous nucleation (in the bulk solution).
2. Form II and Form III are nucleated by homogeneous and heterogeneous nucleation, respectively. An increase in the surface area will affect the heterogeneous nucleation, and there should be no influence on the homogeneous nucleation of Form II. These first and second routes are not consistent with the experimental results reported here.
3. Forms II and III are nucleated by heterogeneous and homogeneous nucleation, respectively.
The pre-exponential factor, A in eqn (2), is proportional to the nuclei centre, which in homogeneous nucleation is one cluster molecule and in heterogeneous nucleation is a number of foreign particles. With a foreign surface where the heterogeneous nucleation occurs, if the foreign surface area increases, the pre-exponential factor of the heterogeneous nucleation of Form II will also increase. Compared with the heterogeneous nucleation of Form II, the homogeneous nucleation rate of Form III should remain the same and, therefore, the percentage of occurrence of the polymorph decreases. However, in most experiments with the tin surface, where both forms of CBZ appear, Form III crystallised on the tin surface and Form II crystals were located on the bottom of the vials. If homogeneous nucleation of Form III happens, the nuclei formed in the solution will settle on the bottom too which cannot transform to Form II, which is not consistent with the experimental observation.
4. Form II and Form III are both from heterogeneous nucleation.
An increase in the foreign surface area leads to an increase in the pre-exponential factor and nucleation rate of the heterogeneous nucleation of Form II. At the same time, the heterogeneous nucleation rate of Form III should also increase, but experimental results show a decrease in the possibility of Form III formation, indicating that the nucleation rate of Form III increases to a lesser extent than that of Form II. Comparing the values of the heterogeneous pre-exponential factors of Form II and Form III is very complicated. The kinetics is dependent on many factors, such as temperature, molecular mobility, etc.,39,40 but the nucleation process and kinetics are still not fully understood. The assumption that heterogeneous nucleation occurs for both Form II and Form III in the solution with foreign surfaces is most probable. However, without accurate induction time for each polymorph in the solution, it is very difficult to understand the process. These nucleation processes need to be further investigated by determining the thermodynamic and kinetic parameters of heterogeneous nucleation and analysing the relationship between the solute and the solvent or solute with foreign surfaces.
Conclusions
Crystallisation experiments involving carbamazepine in ethanol were performed in glass vials with and without foreign surfaces, glass, PTFE or tin. Higher supersaturation and larger foreign surface areas both result in a higher percentage of unstable Form II and a lower percentage of stable Form III formation. In the range of the experimental conditions, the influence on the polymorph is in the order: foreign surface properties > foreign surface areas > degrees of supersaturation.Form III was found to nucleate more preferentially on the surface of tin than those of PTFE and glass in the same solution. The percentage of obtaining Form III on the tin surface remained the same with the increase in surface area at equal supersaturation. A means of selectively crystallising two polymorphs of carbamazepine based on the surface properties is presented.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the EPSRC (EP/N015916/1) for funding.References
- N. Variankaval, A. S. Cote and M. F. Doherty, AIChE J., 2008, 54, 1682–1688 CrossRef CAS.
- J. V. Parambil, S. K. Poornachary, R. B. H. Tan and J. Y. Y. Heng, J. Cryst. Growth, 2017, 8, 84–90 Search PubMed.
- S. Romero, B. Escalera and P. Bustamante, Int. J. Pharm., 1999, 178, 193–202 CrossRef CAS PubMed.
- Y. Kobayashi, S. Ito, S. Itai and K. Yamamoto, Int. J. Pharm., 2000, 193, 137–146 CrossRef CAS PubMed.
- D. K. Bučar, R. W. Lancaster and J. Bernstein, Angew. Chem., Int. Ed., 2015, 54, 6972–6993 CrossRef PubMed.
- H. Yang and Å. C. Rasmuson, Fluid Phase Equilib., 2014, 376, 69–75 CrossRef CAS.
- H. Yang and Å. C. Rasmuson, Fluid Phase Equilib., 2015, 385, 120–128 CrossRef CAS.
- D.-J. Lee, S. Lee and I. W. Kim, Int. J. Mol. Sci., 2012, 13, 10296–10304 CrossRef CAS PubMed.
- C. Capacci-Daniel, K. J. Gaskell and J. A. Swift, Cryst. Growth Des., 2010, 10, 952–962 CAS.
- A. J. Page and R. P. Sear, Phys. Rev. Lett., 2006, 97, 065701 CrossRef PubMed.
- Y. X. Liu, X. J. Wang, J. Lu and C. B. Ching, J. Phys. Chem. B, 2007, 111, 13971–13978 CrossRef CAS PubMed.
- J. V. Parambil, S. K. Poornachary, R. B. H. Tan and J. Y. Y. Heng, CrystEngComm, 2014, 16, 4927 RSC.
- J. V. Parambil, S. K. Poornachary, S. J. Hinder, R. B. H. Tan and J. Y. Y. Heng, CrystEngComm, 2015, 17, 6384–6392 RSC.
- U. V. Shah, C. Amberg, Y. Diao, Z. Yang and J. Y. Y. Heng, Curr. Opin. Chem. Eng., 2015, 8, 69–75 CrossRef.
- Y. Sun, L. Zhu, K. L. Kearns, M. D. Ediger and L. Yu, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5990–5995 CrossRef CAS PubMed.
- N. V. Dziomkina and G. J. Vancso, Soft Matter, 2005, 1, 265 RSC.
- J.-B. Arlin, L. S. Price, S. L. Price and A. J. Florence, Chem. Commun., 2011, 47, 7074 RSC.
- R. K. Harris, P. Y. Ghi, H. Puschmann, D. C. Apperley, U. J. Griesser, R. B. Hammond, C. Ma, K. J. Roberts, G. J. Pearce, J. R. Yates and C. J. Pickard, Org. Process Res. Dev., 2005, 9, 902–910 CrossRef CAS.
- R. J. Behme and D. Brooke, J. Pharm. Sci., 1991, 80, 986–990 CrossRef CAS PubMed.
- F. U. Krahn and J. B. Mielck, Pharm. Acta Helv., 1987, 62, 247 CAS.
- R. C. Kelly and N. Rodríguez-Hornedo, Org. Process Res. Dev., 2009, 13, 1291–1300 CrossRef CAS.
- F. L. Nordström, M. Svärd, B. Malmberg and Å. C. Rasmuson, Cryst. Growth Des., 2012, 12, 4340–4348 Search PubMed.
- K. Sypek, I. S. Burns, A. J. Florence and J. Sefcik, Cryst. Growth Des., 2012, 12, 4821–4828 CAS.
- W. Liu, H. Wei, J. Zhao, S. Black and C. Sun, Org. Process Res. Dev., 2013, 17, 1406–1412 CrossRef CAS.
- C. P. M. Roelands, S. Jiang, M. Kitamura, J. H. ter Horst, H. J. M. Kramer and P. J. Jansens, Cryst. Growth Des., 2006, 6, 955–963 CAS.
- S. Teychené and B. Biscans, Cryst. Growth Des., 2008, 8, 1133–1139 Search PubMed.
- S. Liang, X. Duan, X. Zhang, G. Qian and X. Zhou, RSC Adv., 2016, 6, 74700–74703 RSC.
- A. Getsoian, R. M. Lodaya and A. C. Blackburn, Int. J. Pharm., 2008, 348, 3–9 CrossRef CAS PubMed.
- G. He, A. B. H. Wong, P. S. Chow and R. B. H. Tan, J. Cryst. Growth, 2011, 314, 220–226 CrossRef CAS.
- S. K. Rhee, J. Mater. Sci., 1977, 12, 823–824 CrossRef CAS.
- A. J. Kinloch, Adhesion and Adhesives: Science and Technology, 1987 Search PubMed.
- W. R. Tyson and W. A. Miller, Surf. Sci., 1977, 62, 267–276 CrossRef CAS.
- J. Bernstein, Cryst. Growth Des., 2011, 11, 632–650 CAS.
- T. Lapidot and J. Y. Y. Heng, Ind. Eng. Chem. Res., 2016, 55, 11475–11479 CrossRef CAS.
- S. Aitipamula and A. Nangia, Polymorphism: Fundamentals and Applications in Supramolecular Chemistry: From Molecules to Nanomaterials, John Wiley & Sons Ltd., 2012, DOI:10.1002/9780470661345.smc114.
- J. J. De Yoreo and P. G. Vekilov, Biomineralization, 2003, 54, 57–93 CAS.
- M. M. Lowes, M. R. Caira, A. P. Lötter and J. G. Van der Watt, J. Pharm. Sci., 1987, 76, 744–752 CrossRef CAS PubMed.
- J. P. Reboul, B. Cristau, J. C. Soyfer and J. P. Astier, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1981, 37, 1844–1848 CrossRef.
- R. J. Davey, S. L. M. Schroeder and J. H. ter Horst, Angew. Chem., Int. Ed., 2013, 52, 2166–2179 CrossRef CAS PubMed.
- H. Yang and Å. C. Rasmuson, Cryst. Growth Des., 2013, 13, 4226–4238 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ce01317e |
| This journal is © The Royal Society of Chemistry 2017 |