Switching of the products by changing the size and shape of catalytic nanoparticles during CVD growth of MoS2 nanotubes†
Abstract
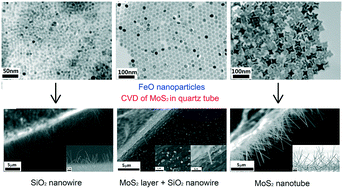
Nanowires of layered materials are important because they exhibit the highest sensitivity as electrically-detecting chemical sensors. MoS2 nanowires have been synthesized by a catalytic chemical vapor deposition method on silicon substrates drop-coated with FeO nanoparticles of different shapes. Switching of the products (MoS2 nanowires to SiO2 nanowires) has been observed when the shapes and sizes of the FeO nanoparticles changed. MoS2 nanowires were grown in the presence of six-horned octahedral nanoparticles, whereas SiO2 nanowires were formed in the presence of spherical nanoparticles. Their morphology, crystal structure and elemental composition have been fully investigated to elucidate the growth mechanism of the nanowires. The kinetics of the grown SiO2 and MoS2 nanowires are competing, giving rise to the observed switching.


 Please wait while we load your content...
Please wait while we load your content...