Short two-armed lanthanide-binding tags for paramagnetic NMR spectroscopy based on chiral 1,4,7,10-tetrakis(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane scaffolds†
Abstract
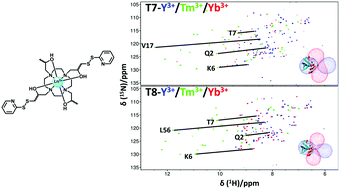
A new pair of enantiomeric two-armed lanthanide-binding tags have been developed for paramagnetic NMR studies of proteins. The tags produce large and significantly different paramagnetic effects to one another when bound to the same tagging site. Additionally, they are less sensitive to sample pH than our previous two-armed tag designs.


 Please wait while we load your content...
Please wait while we load your content...