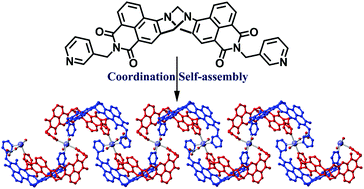
Supramolecular coordination polymers using a close to ‘V-shaped’ fluorescent 4-amino-1,8-naphthalimide Tröger's base scaffold†
Abstract
A V-shaped 4-amino-1,8-naphthalimide derived dipyridyl ligand comprising the Tröger's base structural motif has been synthesised and subsequently used in the formation of two new supramolecular coordination polymers.
- This article is part of the themed collection: Chemosensors and Molecular Logic


 Please wait while we load your content...
Please wait while we load your content...