Liquid-to-gel transition for visual and tactile detection of biological analytes†
Abstract
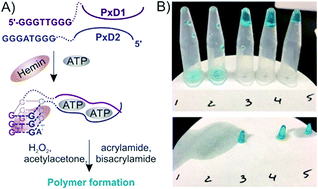
So far all visual and instrument-free methods have been based on a color change. However, colorimetric assays cannot be used by blind or color-blind people. Here we introduce a liquid-to-gel transition as a general output platform. The signal output (a piece of gel) can be unambiguously distinguished from liquid both visually and by touch. This approach promises to contribute to the development of an accessible environment for visually impaired persons.


 Please wait while we load your content...
Please wait while we load your content...