Sydnone–alkyne cycloaddition: applications in synthesis and bioconjugation
Abstract
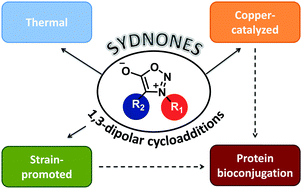
Sydnones are among the most popular mesoionic compounds studied so far for cycloaddition reactions. However, despite their good chemical stability and versatility, only a limited number of research groups have worked on their chemistry and use in organic synthesis. This feature article aims at providing an overview of the most recent developments in sydnone–alkyne cycloadditions, with particular attention on the strategies that allow us to achieving high regiocontrol and milder reaction conditions. The recent discovery that this dipole is able to undergo click and biorthogonal reactions with cycloalkynes may stimulate renewed interest from the scientific community. Given the high potential and flexibility of this family of mesoionics, we believe that major developments are to be expected both in terms of organic synthetic methodologies and biorthogonal chemistry applications in the field of chemical biology.


 Please wait while we load your content...
Please wait while we load your content...