Surface-enhanced Raman spectroscopy: bottlenecks and future directions
Rajapandiyan
Panneerselvam
a,
Guo-Kun
Liu
*b,
Yao-Hui
Wang
c,
Jun-Yang
Liu
a,
Song-Yuan
Ding
a,
Jian-Feng
Li
 ac,
De-Yin
Wu
ac,
De-Yin
Wu
 a and
Zhong-Qun
Tian
a and
Zhong-Qun
Tian
 *a
*a
aState Key Laboratory of Physical Chemistry of Solid Surfaces, iChEM, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: zqtian@xmu.edu.cn
bDepartment of the Environment & Ecology, State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361102, China. E-mail: guokunliu@xmu.edu.cn
cMOE Key Laboratory of Spectrochemical Analysis and Instrumentation, Xiamen University, Xiamen 361005, China
First published on 30th October 2017
Abstract
In this feature article, we discuss in detail developmental bottleneck issues in Raman spectroscopy in its early stages and surface-enhanced Raman spectroscopy (SERS) in the past four decades. We divide SERS research into two different directions with different targets. Fundamental research is extending the limits of SERS to single-molecule, sub-nanometer resolution and femtosecond processes. In contrast, practical research is expanding the range of applications with the aim of providing versatile analytical tools for surface, materials, life, environmental, forensic and food sciences and also commercial instruments for use in daily life. In the second direction there have continually been many complex bottlenecks to be overcome. We attempt to enumerate the key issues in detail and also describe the achievements made to overcome the bottlenecks. In the last, but not least important part, we discuss the remaining bottlenecks and possible strategies for overcoming them to enable SERS to be an even more powerful and versatile technique.
Dr Guo-Kun Liu (1st from right, front) is an associate professor in the State Key Laboratory of Marine Environmental Science, College of the Ecology and Environment, Xiamen University. He received his BSc in chemical education at Soochow University in 1999 and his PhD in physical chemistry at Xiamen University in 2006. His current work focuses on extending surface-enhanced Raman spectroscopy and electrochemistry from frontier research to practical applications in the fields of food science, environmental monitoring and marine science. |
Mr Yao-Hui Wang (2nd from right, back) received his BSc from the Department of Chemistry at Dezhou University in 2013. He is currently a PhD candidate under the supervision of Prof. Jian-Feng Li in the Department of Chemistry at Xiamen University. His research focuses on surface-enhanced Raman spectroscopy and single-crystal electrochemistry. |
Dr Jun-Yang Liu (2nd from right, front) is a postdoctoral fellow in the Department of Chemical and Biochemical Engineering at Xiamen University, China. He obtained his BSc in 2010 and his PhD under the supervision of Prof. Zhong-Qun Tian in 2016 at Xiamen University. Currently, his main research interests include single-molecule electronics, SERS, plasmonics and micro- and nanofabrication. |
Dr Song-Yuan Ding (3rd from right, front) received his BSc in Chemical Physics at the University of Science and Technology of China in 2005 and his PhD in Chemistry under the supervision of Prof. Zhong-Qun Tian at Xiamen University in 2012. He is a Research Fellow in the Collaborative Innovation Center of Chemistry for Energy Materials (iChEM) at Xiamen University. Currently, his research interests include surface-enhanced Raman spectroscopy for general materials, AFM-based infrared and Raman nanospectroscopy and imaging, ab initio interfacial electrochemistry and the theory of catalytic molecular assembly. |
Dr Jian-Feng Li (1st from left, back) is a full professor of Chemistry at Xiamen University. He received his BSc in Chemistry from Zhejiang University in 2003 and his PhD in Chemistry under the supervision of Prof. Zhong-Qun Tian at Xiamen University in 2010. He is a principal inventor of SHINERS (shell-isolated nanoparticle-enhanced Raman spectroscopy). Currently, his research interests include surface-enhanced Raman spectroscopy, core–shell plasmonic nanostructures, (single-crystal) electrochemistry, and surface catalysis. |
Dr De-Yin Wu (2nd from left, front) received his BSc at Shaanxi Normal University in 1992 and his PhD in Physical Chemistry at Sichuan University in 1998. Currently, he is a full professor in the Department of Chemistry, College of Chemistry and Chemical Engineering and State Key Laboratory of Physical Chemistry of Solid Surfaces, Xiamen University. His research areas focus on the theories of surface-enhanced Raman spectroscopy, adsorption and electron transfer at electrochemical interfaces and charge transport in molecular electronics. Currently, his research interest is surface plasmon resonance-photoelectrochemical reactions on noble metal nanostructures. |
Dr Zhong-Qun Tian (1st from left, front) is a full professor of Chemistry at Xiamen University, China. He obtained his BSc at Xiamen University in 1982 and his PhD under the supervision of Prof. Martin Fleischmann at the University of Southampton in 1987. Professor Tian is a Member of the Chinese Academy of Sciences and the Elected President of the International Society of Electrochemistry. Currently, his main research interests include SERS, spectroelectrochemistry, nanochemistry, plasmonics, and catalytic molecular assembly. |
Introduction
In 1928, two Indian physicists, namely Chandrasekhara Venkata Raman and Kariamanickam Srinivasa Krishnan, discovered “a new type of secondary radiation” termed Raman scattering (inelastic scattering).1 After this discovery, Raman spectroscopy was developed into a powerful analytical tool for providing important information about the chemical structure and bonding of target analytes.2–4 Unlike infrared spectroscopy, Raman spectroscopy does not suffer from strong interference from CO2 and water under ambient conditions. However, Raman scattering events are inherently weak (∼1 of 1 million incident photons). Therefore, very low detection sensitivity was a developmental bottleneck of Raman spectroscopy, in contrast to those of infrared absorption or fluorescence emission spectroscopy.5 In addition, the lack of intense stable sources and sensitive detecting technology limited the application of Raman spectroscopy in various fields. This is the main reason why Raman spectroscopy was not widely adopted by the scientific community for almost three decades after its invention.When the laser was invented in the 1960s, the detection limit of Raman spectroscopy was significantly increased by high-intensity laser sources and improved detecting technology. However, simply increasing the laser power by up to five or more orders of magnitude is not a practical way of increasing the sensitivity of Raman spectroscopy, because the tested samples will be damaged or burned. Owing to its intrinsically weak scattering process, Raman spectroscopy was not used for surface science, electrochemical studies, or trace analysis.6 The intensity of the incident photon flux of typically 108 to 1010 is not sufficient to detect a surface adsorbate with (sub)monolayer coverage.
The first surface Raman spectra that were dependent on the electrochemical potential were surprisingly observed from pyridine molecules adsorbed on a roughened silver electrode by Fleischmann, Hendra, and McQuillan in 1974.7 This achievement stemmed from their pioneering work on the application of Raman spectroscopy to electrochemistry. In fact, this was the first SERS measurement, although it was not recognized as such at that time.
Van Duyne and Jeanmaire soon carefully devised a procedure for measuring the surface enhancement factor, in which the signal intensity of a specific molecule on a surface is compared with that of the same molecule in solution. They discovered that the enhancement factor was of the order of 105–106. They explained that the roughened electrode surface created an enhanced electric field within the electrochemical double-layer region, which thus increased the Raman scattering cross-section of adsorbed pyridine molecules. After a protracted review process, which was presumably due to the reluctance of reviewers to believe an unorthodox concept of surface enhancement, their paper was eventually published in 1977.8 Independently, Creighton and Albrecht published a paper in the same year,9 which provided evidence of the giant Raman effect, but they explained the enhancement to be due to the resonance Raman-like effect of the surface adsorbate.
In 1978, Moskovits first explained the effect of surface plasmons on roughened silver electrodes (a collective oscillatory mode of conduction electrons from metal nanostructures) on SERS enhancement and also predicted that the same effect may occur with silver (Ag) and copper (Cu) colloids covered with an adsorbate.10 This prediction was successfully verified by Creighton et al. using silver and gold (Au) colloids in 1979.11 Their approaches significantly expanded the area of study from roughened electrode surfaces to metal colloids, which thus indicated the main avenue for the development of SERS based on plasmonic nanostructures. (In 1857, Faraday first envisaged the plasmonic properties of thin Au films prepared from colloidal Au NPs: upon mechanical pressure, the film experienced a reversible color change from bluish-purple to green.12) This ‘giant Raman effect’ was dubbed by Van Duyne as surface-enhanced Raman scattering (SERS).13
It is now well known that SERS is a phenomenon in which the Raman signals of molecules are enormously enhanced when they are very close to certain SERS-active nanostructures.14 Nanostructures of free-electron-like metals (e.g., Au, Ag, and Cu) could massively concentrate incident optical fields at locations where the analyte of interest is present and could also enhance the radiation efficiency of the oscillating dipole source of the analyte at the Raman scattering frequencies. This two-step enhancement is mainly due to the excitation of surface plasmons (SPs) of the metal nanostructures by incident light in the far field and Raman scattering of oscillating dipole radiation in the near field.15–18 By this two-step enhancement process, the total Raman intensities of analytes can usually be increased by up to 6 to 8 orders of magnitude.19 Besides the electromagnetic (EM) enhancement mentioned above, a non-EM contribution such as chemical enhancement (CE) may also take place. EM enhancement plays a predominant role in the SERS effect, whereas in most cases the CE mechanism contributes a far smaller SERS enhancement effect than that due to EM.20 Furthermore, EM enhancement is a long-range effect restricted to the evanescent field (normally less than 5 nm), which decays exponentially with the distance between the target molecule and the SERS-active substrate, whereas CE is a short-range effect on the angstrom scale with a direct interaction between the target and the substrate.
The discovery of SERS soon created an upsurge of interest, because SERS can detect ultralow concentrations of target molecules on plasmonic metal nanostructures, even at a single-molecule level.21 Thus, SERS has attracted widespread interest owing to its ultrahigh sensitivity and molecular specificity in chemical analysis, surface analysis and biomolecular analysis.22–24 However, in the 1980s researchers gradually realized that the disadvantages of SERS could not be fully overcome to remove the developmental bottleneck in surface Raman spectroscopy because of its versatility for a wide range of applications (see below). Moreover, SERS differs in several ways from ordinary Raman spectroscopy owing to the differences in its surface selection rules and enhancement effects.25,26 For an extended overview of the SERS phenomenon, we refer readers to the excellent reviews by Aroca,27 Kerker,28,29 Etchegoin and Le Ru,30 Metiu,31,32 Moskovits,33,34 Otto,35 Ozaki,20 Schatz,36,37 Smith,38 Tian,22,39,40 and Van Duyne et al.14,41,42 (the authors’ names are listed in alphabetical order).
1. Research directions and developmental bottlenecks in SERS
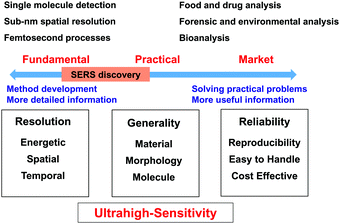
To obtain a comprehensive picture, we divide SERS studies into two different research directions, namely, fundamental research and practical research, as shown in Fig. 1. Fundamental research is focused on the development of methods to extend the limits of SERS to single-molecule and sub-nanometer resolution and femtosecond processes to enable, for example, single-site chemical reactions43,44 and molecular electronics.45–47 In contrast, practical research is focused on various potential applications. The key target is that SERS should become a versatile analytical tool and methodology for surface, materials, life, environmental, forensic and food sciences and then further develop into a commercial instrument that is widely used in medicine, energy, agriculture, pollution, security and criminal investigation.48–50To achieve these goals, Raman studies should achieve ultrahigh sensitivity in a wide range of target systems. In this regard, in 1997 Nie and Emory51 and Kneipp et al.52 independently made spectacular breakthroughs in the SERS field by reporting single-molecule SERS measurements. SERS became the first vibrational spectroscopy technique to attain ultrahigh sensitivity on the single-molecule level. This observation rekindled the interest of several branches of the scientific community in SERS owing to this enormous enhancement and the possibility of detecting single molecules.
In the direction of fundamental research, significant progress has been made, and researchers have attained single-molecule detection with sub-nm spatial resolution. However, in the other direction, researchers are unable to apply SERS as a versatile tool for the whole scientific community and a general and reliable analytical technique for daily life and society. Unfortunately, the scope of application of SERS is limited by a few serious obstacles to its application to a wide range of materials, surfaces and morphologies and a great variety of molecules. For instance, from fundamental research it is known that substrates with heterogeneous but locally very strong enhancement are preferred to enable the detection of single molecules, whereas for practical applications a homogeneous signal enhancement is desired. Even now, it is difficult to develop easy-to-use and low-cost SERS substrates and instruments with high stability and reproducibility. In the following sections, we will attempt to survey and discuss the difficulties and challenges in the SERS field with the main aim of improving and broadening the range of applications of SERS.
1.1 Bottlenecks in SERS in fundamental research
SERS enhancement effects are inherently confined to the morphology and optical properties of SERS-active nanostructures.53 Notably, free-electron metal nanostructures (e.g., Ag, Au, and Cu) are preferred as SERS substrates owing to their surface plasmon properties in the visible region. Ag and Au are highly preferred for SERS studies because of their electronic structure, surface morphology, and interactions with target analytes, whereas Cu is more reactive and exhibits weak SERS signals.54 However, other transition metals such as Pd, Pt, Rh, Ru, Co, Fe, and Ni are widely used in electrochemistry, corrosion, catalysis, and surface science, but exhibit weak SERS enhancement in the range of 101–103.55It should be noted that in SERS, metal nanostructures play a dual role as a signal amplifier and a host for target molecules, which restricts the versatility of SERS as a tool. To achieve the maximum enhancement, probe molecules should be directly attached to metal nanostructures, in particular in the so-called hotspot, to experience the maximum enhancement in the local field, i.e., EM enhancement. Besides, a possible charge transfer effect between bare metal nanostructures and adsorbates, i.e., CM enhancement, may also contribute to the total Raman enhancement. In summary, the SERS process involves complicated coupled three-body interactions among photons, molecules, and nanostructures.35,36,56
SERS with close interactions between bare metal nanostructures and target molecules can be described as contact-mode SERS. The contact mode exhibits excellent Raman enhancement in various applications for the detection of trace molecules dispersed in solution or air. However, contact-mode SERS suffers from several limitations if we want to characterize the surfaces and interfacial structures of materials and biosamples. In some cases, interfacial charge transfer may occur between bare metal nanostructures and materials (Pt, Co, or Ni) to be studied. Close contact between noble metal nanostructures and target molecules may result in a photochemical reaction under intense laser illumination. In general, metal nanostructures are susceptible to contamination, which in some cases may lead to an erroneous interpretation of SERS signals.57,58 Thus, it is important to understand the nature of probe molecules and their interaction with metal nanostructures.
Taking into account the principle of SERS, only free-electron metal nanostructure surfaces generate giant SERS enhancements. Therefore, the extension of SERS to various morphologies, such as flat surfaces, single crystals, semiconductors and soft materials, i.e., morphological generality, is another key issue in SERS. For instance, a flat metal substrate cannot support a strong enhancement in the local field unless an external optical element such as a prism is coupled with a flat Au or Ag film to support a propagating surface plasmon. This coupling configuration for Raman measurements is termed attenuated total reflection (ATR-Raman). By means of the ATR-Raman configuration, the Raman enhancement of probe molecules adsorbed on the surfaces of a flat Au film is only two orders of magnitude.59,60 For other transition metal films, the enhancement factor of ATR-SPP would be much less. Thus, more effort should be made to significantly improve the sensitivity of SERS on flat surfaces such as single-crystal surfaces.
1.2 Bottlenecks in SERS in commercial market
It seems surprising that after four decades SERS has not yet been successfully commercialised and widely used. As shown in Fig. 1, to employ SERS techniques in daily life SERS substrates and techniques should be standardized and meet several requirements: (1) stability of SERS substrates in ambient environments; (2) both substrate-to-substrate and spot-to-spot reproducibility; and (3) selectivity and molecular generality of SERS measurements. We refer the reader to the excellent remarks by Natan for a detailed discussion of scientific standards in SERS.61 Besides, SERS substrates should be cost-effective and easy to handle. In general, SERS substrates can be classified into two major categories: structured surfaces and metal nanostructures (colloids).62 For commercial applications, SERS active metal nanostructures should have a long shelf life (>1 year at least) with significant SERS activity. The relative standard deviation of SERS measurements is typically ∼15–20% but has been significantly reduced in recent years with the development of nanoscience. In general, the morphology and stability of nanomaterials greatly affect the sensitivity, reproducibility and efficiency of any analytical technique because controlling the morphology and assembly of nanomaterials on a nanometer scale is a common challenging task in nanoscience and nanotechnology.63Structured surfaces are susceptible to surface oxidation and contamination once exposed to ambient environments, and one must be very careful when handling them for SERS measurements. Importantly, it is difficult to prepare nanopatterned surfaces with controllable nanogaps that are smaller than 2 nm. Therefore, the SERS enhancement of structured surfaces is typically much less than that of metal nanoparticles prepared by wet-chemistry methods. Metal nanoparticles can be used for SERS studies because they can be stored as colloidal solutions to prevent oxidation and contamination. However, metal colloids are susceptible to aggregation, and surface capping agents may act as contaminants and thus contribute to the Raman signals and interfere with the SERS signals of probe molecules. Thus, the lack of stability, selectivity, sensitivity and reproducibility has severely impeded the standardization and practical utilization of SERS substrates for practical applications.
In summary, the abovementioned bottleneck problems have hindered the widespread application of SERS in both fundamental research and practical research in various fields. However, the number of publications dealing with SERS has tremendously increased in the past few decades. Apparently, statistics from the Institute for Scientific Information (ISI) database show that the total number of SERS-related publications (using surface enhanced Raman as keywords) in 1980, 2000, 2012, and 2016 were 86, 453, 2797, and 4136, respectively. To be frank, most of these publications were merely related to plasmonic nanomaterials and simply reported their potential for SERS applications. The threshold for entering the SERS field is quite low, because the synthesis of Au and Ag nanoparticles is very easy. These studies have not contributed to the real development of SERS. Therefore, it is necessary to give a comprehensive analysis and discuss how to overcome the developmental bottlenecks in SERS.
2. Overcoming the bottlenecks in SERS (since the 1980s)
For the past few decades, many groups, ourselves included, have made great attempts to eliminate the key limitations of SERS to extend its range of applications in other scientific communities and the commercial market. However, a thorough review of new SERS substrates and their preparation is beyond the scope of this review. Thus, we restrict ourselves to the discussion of three exemplary strategies: (1) the ‘borrowing SERS’ strategy, (2) tip-enhanced Raman spectroscopy (TERS), and (3) shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS).2.1 ‘Borrowing SERS’ strategy and other features/strategies
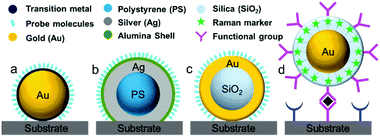
During the 1980s, structurally ill-defined metal nanostructures decisively affected the reproducibility and reliability of SERS techniques and limited the fundamental understanding and application of SERS in various fields such as surface/interface, materials and life sciences. To overcome issues with material generality, in 1983 Van Duyne et al. initially proposed an approach termed the ‘borrowing SERS’ strategy to acquire Raman signals of molecules on SERS-inactive materials such as n-GaAs electrodes.64 The preparation of SERS substrates comprises a tortuous pathway and is primarily focused on well-controlled metal nanostructures and core–shell nanoparticles rather than electrochemically roughened surfaces.55,65,66 For instance, Fleischmann et al. and Weaver et al. extended the range of application of SERS using SERS-active nanostructures coated with transition metals.67,68 Several attempts have been made to exploit other transition metals such as Pt, Fe, Ru, Rh, Co, Ni, and Pd electrodes, but the Raman enhancement was very limited and ranged from one to three orders of magnitude.55,69Fig. 2 shows four different strategies used to prepare SERS substrates for detecting a wide range of target molecules using the ‘borrowing SERS’ strategy. With the aid of the long-range effect of EM enhancement from the SERS-active metal core, high-quality SERS spectra have been acquired from core–shell nanostructures (SERS-active core coated with an ultrathin transition metal shell). However, to obtain a high SERS enhancement, a pinhole-free ultrathin shell is an important prerequisite for transferring the EM enhancement from the SERS-active metal core and avoiding direct contact between core particles and analyte molecules.70Another interesting method has been employed by several groups for multiplexed assays. In this method, strongly resonant molecules (e.g., dyes) are employed as Raman markers by being directly attached to the surface of SERS-active nanoparticles (Au or Ag).69 Furthermore, nanoparticles tagged with Raman markers are modified with a silica, polyelectrolyte or polymer shell to provide chemical and mechanical stability for nanoparticles and Raman tags.71,72 Interestingly, the shell bears various functional groups to interact with target molecules. Importantly, the Raman signals obtained from the Raman markers are used for the related analysis, irrespective of the Raman signals of target molecules and their distance from the nanoparticles. The ultrahigh sensitivity and feasibility for use in multiplexed assays are considered to be two striking features, so that this strategy can be employed for various practical applications.
To a certain extent, the problem of material generality has been resolved by introducing the ‘borrowing SERS’ strategy. Together with significant developments in instrumentation and technology (e.g., confocal microscopes, holographic filters, collection optics, and CCD detectors), Raman spectroscopy has accumulated abundant information in both fundamental and practical research.73–75
2.2 Tip-enhanced Raman spectroscopy (TERS)
As mentioned previously, the applications of SERS have been restricted by materials, morphologies and molecular generality. In addition, the spatial resolution of Raman spectroscopy is limited to the sub-micron regime because of the optical diffraction limit. To overcome the limitations of SERS, the concept of tip-enhanced Raman scattering (TERS) was first proposed by Wessel76 in 1985. In 2000, four groups77–80 realized the significance of TERS independently. In principle, the TERS technique comprises a combination of scanning probe microscopy, such as atomic force microscopy (AFM), scanning tunnelling microscopy (STM) or shear force microscopy (SFM), and Raman spectroscopy.81TERS employs a sharp tip that is composed of or coated with Au or Ag. In some cases, an Au or Ag nanoparticle or nanostructures can be attached to the AFM or SFM probe.82 The sharp gold or silver SPM tip generates a localized enhanced electromagnetic field under excitation by a suitable source. The enhanced electromagnetic field can enhance the Raman signals of adsorbed probe molecules and surfaces by up to six orders of magnitude.83 In TERS, strong electromagnetic coupling between the metal tip and the metal substrate is essential for reliable TERS imaging.
The TERS technique can be employed to understand fundamental processes in various fields such as surface science, materials science, physics and chemistry.83,84 For instance, Van Duyne et al. explained the origin of fluctuations in relative intensity in single-molecule Raman experiments using both single-molecule tip-enhanced Raman spectroscopy and time-dependent density functional theory calculations.85 The ability of TERS to determine molecular properties of the excited state of a molecule can provide clues to the interactions between adsorbates and surfaces.
During the past 16 years, the spatial resolution of TERS has been improved drastically from several tens of nanometres to the sub-nanometre range. For instance, Kawata et al. reported a resolution of 1.7 nm in the chemical analysis of carbon nanotubes by STM-TERS imaging in ambient conditions.86 Ren et al. investigated the electronic and catalytic properties of a bimetallic surface by TERS and achieved a spatial resolution of 3 nm.87 They designed atomically well-defined Pd (sub-monolayer)/Au(111) as a model bimetallic catalyst. STM images and TERS spectra of the model catalyst were obtained simultaneously using phenyl isocyanide (PIC) as a probe molecule (Fig. 3a). The intensities of the three main peaks of PIC (1165, 1590 and 1995 cm−1) are plotted as a function of the tip position in Fig. 3b, and were found to be closely correlated with the fine structure of the catalyst. These findings demonstrate that TERS is ideally suited to the study of defects, step edges and perimeter interfaces of various catalytic materials and can play a crucial role in determining catalytic performance.
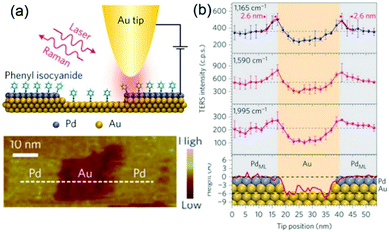 | ||
| Fig. 3 (a) Schematic of an STM-based TERS configuration using Au tips and Pd/Au(111) substrates with phenyl isocyanide (PIC) on the surface. (b) Top three panels: Plots of intensities of the three main TERS peaks (1165, 1590 and 1995 cm−1) against the tip position. Bottom panel: topographic height profile of the surface along the dashed line in (a), superimposed with the atomic model of the surface atoms. The size of atoms is to scale on the y axis but not to scale on the x axis. Purple, Pd; yellow, Au. The error bars indicate the standard deviations from three measurements. Reproduced from ref. 87 with permission from Nature Publishing Group. | ||
Zhang et al. dramatically improved the spatial resolution of TERS by employing UHV sample preparation and measurements to improve the cleanliness of the surface and the stability of the tip.88 The authors reported a spatial resolution of as low as the sub-nanometre scale (below 1 nm) in the Raman signals of meso-tetrakis (3,5-di-tert-butylphenyl) porphyrin (H2TBPP) on the surface. Fig. 4 illustrates the configuration, TERS spectra, and TERS mapping of a single molecule.88 Several theoretical studies have been reported to interpret the sub-nanometre resolution achieved in this landmark breakthrough work.82,89,90 Although the mechanism underlying this unprecedented spatial resolution of STM-TERS mapping is not yet completely clear or well accepted, this work will significantly motivate the further development of theories, as well as applications, in surface chemistry and physics and molecular electronics.91
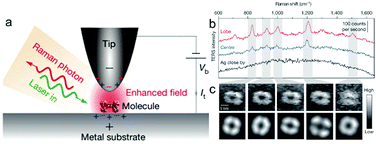 | ||
| Fig. 4 (a) Schematic of tunnelling-controlled TERS in a confocal-type side illumination configuration, in which Vb is the bias applied to the sample and It is the tunnelling current. (b) Representative single-molecule TERS spectra recorded on the lobe (red) and centre (blue) of a molecule lying flat on Ag(111). The TERS spectrum on bare Ag about 1 nm away from the molecule is also shown (black) (120 mV, 1 nA, 3 s). (c) The top panels show the experimental TERS mapping of a single molecule derived from different Raman peaks (23 × 23, ∼0.16 nm per pixel), which was processed from all the individual TERS spectra acquired at each pixel (120 mV, 1 nA, 0.3 s; image size: 3.63, 3.62 nm). The bottom panels show a theoretical simulation of the TERS mapping. Reproduced from ref. 88 with permission from Nature Publishing Group. | ||
In practical research as well, TERS has been employed to examine materials with large cross-sections such as carbon allotropes, semiconductors, polymers, solar cell materials, nucleic acids, bacteria, viruses, cells, and proteins.92–95 For example, Deckert et al. employed a TERS technique to detect the sequences of single RNA strands96 and monitor photocatalytic reactions on the nanoscale.97 In particular, TERS makes a significant difference by utilizing a sharp metallic tip, which only acts as a signal amplifier. To a certain extent, TERS offsets the limitations of SERS by detecting target molecules on any surface or morphology without direct contact. However, in some cases, the bare TERS tip encounters interference from the matrix or target molecules and has problems with sensitivity, because the TERS enhancement is limited to a single tip and a few molecules.98
2.3 Shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS)
To circumvent the shortcomings of SERS and TERS, in 2010 our group combined the concepts of TERS and the ‘borrowing SERS’ strategy to introduce a new method termed “shell-isolated nanoparticle-enhanced Raman spectroscopy” (SHINERS).99 As shown in Fig. 5, SHINERS employs ultrathin shell-isolated nanoparticles, where each nanoparticle serves as a TERS tip to acquire SERS signals with significant sensitivity. SHINERS is becoming increasingly attractive because shell-isolated nanoparticles (SHINs) can spread over any surface or morphology, such as semiconductors, single-crystal surfaces, non-transition metals, fruits, and vegetables, to obtain signals from target analytes.57,100,101 In recent years, researchers have employed SHINERS in the fields of electrochemistry,66,102 heterogeneous catalysis, and environmental analysis.103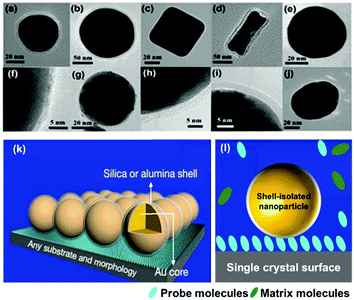 | ||
| Fig. 5 HR-TEM images of various types of SHIN: (a) Au@SiO2 NPs with a 55 nm spherical core, (b) a 120 nm spherical core, (c) a nanocube core, (d) a nanorod core; (e) an Ag@SiO2 NP; (f) a Cu@graphene NP;104 and SHINs with a core of gold and a shell of (g) graphene,105 (h) SiO2, (i) Al2O3, (j) MnO2. (k) Working principle of SHINERS. (l) During SHINERS measurements, SHINs do not suffer from interference due to the presence of matrix molecules in the surrounding medium. (f) Reproduced from ref. 104 with permission from the American Chemical Society; (g) reproduced from ref. 105 with permission from Nature Publishing Group. | ||
Notably, the shell-isolated nanoparticles (SHINs) are coated with ultrathin (2–5 nm) silica or alumina shells, which are chemically and electronically inert. SHINERS provides an excellent research tool for obtaining interfacial molecular information that is unaffected by contamination, aggregation, oxidation, and electronic interactions of nanoparticles with matrix species in the surrounding medium and metallic substrates.75 The shell-isolated mode in SHINERS is different from the contact mode in SERS because the inert uniform thin shell reliably transfers the EM enhancement from the SERS-active core and also avoids electrical contact between the probe surface and metal nanoparticles. In SHINERS, the dual functions of nanoparticles have been effectively isolated with the help of the inert shell material. However, the average SHINERS enhancement factor is lower than the average SERS enhancement factor. Fig. 5 clearly illustrates the working principle of SHINERS on a non-traditional SERS substrate and shows that SHINs do not experience interference from matrix molecules in the surrounding environment but provide Raman information on surface species. Importantly, shell-isolated nanoparticles can be commercialized for fundamental research, because the thin inert shell maintains the SERS activity of the core for almost 1 year and protects the nanoparticles from oxidation, acidic or harsh environments, and aggregation.101 SHINERS has delivered significant information about adsorbates at well-defined single-crystal surfaces with the aid of SHINs.106–111 For instance, we developed a general strategy for monitoring heterogeneous catalytic processes by the fabrication of SHINERS-satellite nanocomposites (Au core–silica shell nanocatalyst-satellite structures) (Fig. 6a).112 The reaction processes and mechanisms of the oxidation of CO over Pd nanocatalysts were revealed by combining in situ SHINERS studies with DFT calculations. Spectroscopic evidence shows that CO would hinder the activation of O2 on a Pd surface, which would lead to low activity at low temperatures (Fig. 6b–d). With an increase in the reaction temperature, Pd oxide will form, on which O2 is then adsorbed and activated to form superoxide and peroxide species, which results in an increase in activity. This study demonstrates that SHINERS can be developed as a standard characterization method for the in situ monitoring of reaction intermediates and the elucidation of reaction mechanisms.112 The abovementioned progress has overcome the generality limitation to some extent, and further developments are desirable to enable SERS to be an even more powerful and versatile technique.
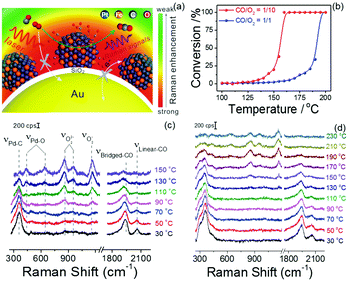 | ||
| Fig. 6 (a) Schematic of SHINERS-satellite strategy for the in situ monitoring of the oxidation of CO on PtFe. The blue, orange, grey and red spheres represent Pt, Fe, C and O atoms, respectively. (b) Catalytic performance of the oxidation of CO over Pd nanocatalysts under different feed conditions. (c and d) In situ SHINERS spectra for the oxidation of CO over Pd nanocatalysts at different temperatures at ratios of CO/O2 = 1/10 and 1/1, respectively. Reproduced from ref. 112 with permission from Nature Publishing Group. | ||
3. Future bottlenecks and perspectives
After over four decades of development, SERS has become the eldest member of the family of SP-based spectroscopic techniques, which is termed as plasmon-enhanced spectroscopy.50 Many new members have been invented such as surface-enhanced second-harmonic generation (SE-SHG),113 surface-enhanced infrared absorption spectroscopy (SEIRA),114 surface-enhanced fluorescence (SEF),115 surface-enhanced hyper-Raman spectroscopy (SE-HRS),116 surface-enhanced sum frequency generation (SE-SFG),117 surface-enhanced coherent anti-Stokes Raman scattering (SE-CARS),118 surface enhanced spatially offset Raman spectroscopy (SE-SORS), tip-enhanced Raman scattering (TERS),119 and shell-isolated nanoparticle-enhanced Raman scattering (SHINERS).120 Thus, developments in nanoscience and instrumentation technology have transformed SERS and the other techniques to enable a next phase with potential applications.For a better understanding of future bottlenecks in detail, we have listed the advantages and disadvantages of SPR, Raman spectroscopy, SERS, TERS, and SHINERS in Table 1. To a great extent, SERS has been applied successfully in fundamental research. Furthermore, it is necessary to extend the uses of SERS to daily life from the viewpoint of practical applications. In the following sections, we discuss in depth future directions for SERS and possible strategies for overcoming the related challenges.
| Surface plasmon resonance | Raman spectroscopy | Random nanostructures (SERS) | Ordered nanostructures (SERS) | TERS | SHINERS | |
|---|---|---|---|---|---|---|
| Energetic resolution |
Poor
(10−2 eV) |
Excellent
(10−4 eV) |
Excellent
(10−4 eV) |
Excellent
(10−4 eV) |
Excellent
(10−4 eV) |
Excellent
(10−4 eV) |
| Spatial resolution |
Good
(300 nm) |
Good
(300 nm) |
Good
(300 nm) |
Good
(300 nm) |
Excellent
(10 nm) |
Good
(300 nm) |
| Temporal resolution |
Excellent
(10−9 s) |
Excellent
(10−9 s) |
Excellent
(10−9 s) |
Excellent
(10−9 s) |
Poor
(0.1 s) |
Excellent
(10−9 s) |
| Morphological generality | Poor | Excellent | Poor | Good | Excellent | Excellent |
| Material generality | Poor | Excellent | Poor | Fair | Excellent | Excellent |
| Molecular generality | Fair | Good | Fair | Good | Fair | Good |
| Sensitivity | Excellent (single molecule) |
Poor
(many molecules) |
Excellent
(single molecule) |
Excellent
(single molecule) |
Excellent (single molecule) |
Good
(sub-monolayer) |
| Reproducibility | Poor | Excellent | Poor | Good | Fair | Good |
| Stability of nanoparticles | Poor | NA | Poor | Poor | Poor | Excellent |
| Resistance to contamination/photoreaction | Poor | NA | Poor | Poor | Poor | Very good |
3.1 Molecular generality
To be truly powerful and versatile for the analysis of surface and trace chemicals, a technique must be capable of detecting a great variety of analytes. To gain deeper insights in fundamental and practical research, SERS has to be pushed to the limit, i.e., to become even more sensitive in order to detect a range of target molecules such as molecules with small cross-sections and weakly adsorbed species with significant sensitivity. However, molecular generality has always been a challenge, taking into account the principles of SERS, which is a limitation that has hardly been overcome. The typical Raman cross-section of a molecule is inherently small at around 10−29–10−32 cm2, which is 106 and 1014 times smaller than those for IR and fluorescence spectroscopy, respectively.121 For the past few decades, Raman spectroscopic techniques have been employed to extract valuable information about molecules with large cross-sections.For instance, electron-rich aromatic molecules such as pyridine can interact with nanoparticle surfaces and exhibit intense Raman signals. However, weakly adsorbed molecules with much smaller Raman cross-sections such as methanol, water, and perchlorate cannot exhibit strong SERS signals.122 It is necessary to obtain interfacial information about these molecules to clearly understand related chemical, electrochemical, or biological processes. Accordingly, molecular generality has always been the most challenging bottleneck of SERS/TERS/SHINERS.
As we mentioned earlier, Raman spectroscopy is not affected by water molecules owing to their small cross-section. However, it is essential to detect surface water molecules when they are co-adsorbed with targeted surface species. Water and some surface species have very weak interactions with metal nanoparticles. For instance, ClO4− and several important anions in electrochemical systems do not strongly interact with the surfaces of Au or Ag electrodes, which leads to very low surface coverage. As a consequence, it is extremely difficult to acquire SERS signals of these anions (Fig. 7). To obtain the whole picture of the studied surface/interface and understand the mechanisms of surface processes, all surface species should be detected and analysed simultaneously. However, at the present stage most SERS studies only focus on the detection of strong adsorbates that have large Raman cross-sections such as bipyridine, rhodamine 6G (R6G) or p-nitrothiophenol (PNTP), etc. In general, the detection of weakly adsorbed molecules/ions with low surface coverage or at trace levels in various fields such as surface, electrochemical, materials, food and forensic science is a long-standing key challenge for SERS/TERS/SHINERS and the other members of this family of techniques.
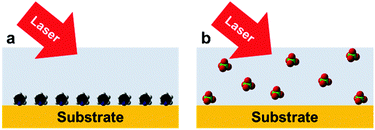 | ||
| Fig. 7 High and low surface coverage of different adsorbates on a substrate surface: (a) strong chemical adsorption; (b) weak physical adsorption. | ||
To detect weakly adsorbed species, one must further increase the selective sensitivity. Several strategies, including specific capture, derivatization and labelling, have been applied to obtain the SERS signals of target molecules directly or indirectly.123,124 Researchers can functionalize the surfaces of SERS-active nanostructures to specifically recognize target molecules. For molecules with small Raman cross-sections, one should further increase the signal-to-noise ratio by using signal-averaging techniques or by increasing the enhancement factor as far as possible by designing highly plasmonic-active nanostructures.
3.2 Nanostructures for plasmon-enhanced Raman spectroscopy
TERS is considered to be an important nanospectroscopic technique for the molecular-level and nanoscale analysis of electrochemical systems related to solar cells, lithium-ion batteries, fuel cells, and corrosion. Therefore, the broader uptake of TERS in electrochemical studies implicitly requires a stable and reproducible metallic tip to achieve the sensitive detection of molecules with small cross-sections. More recently, Ren et al. developed an electrochemical STM cell for electrochemical TERS (EC-TERS) to minimize optical distortion and carry out TERS measurements under well-controlled electrochemical conditions.125 Van Duyne et al. employed EC-TERS to monitor structural changes in Nile blue upon its reduction and at a particular point of interest on the electrode surface.126To improve the sensitivity of TERS, Raschke et al. developed gold tips coupled with a grating and demonstrated plasmonic nanofocusing upon localized excitation of a sample within a range of ∼20 nm.127 To improve the stability of TERS tips, Li et al. proposed a chemical method for preparing shell-isolated TERS tips for shell-isolated tip-enhanced Raman spectroscopy (SITERS), which eliminates the disadvantages associated with a bare metallic tip.128 In the near future, the shell-isolated nanoparticle strategy can be incorporated with other surface-enhanced techniques, such as surface-enhanced infrared spectroscopy,129 surface-enhanced electrochemiluminescence,130 second-harmonic generation and surface-enhanced fluorescence,131 because their enhancement depends on the morphology and stability of nanoparticles.
In the case of SHINERS, the formation of pinhole-free SHINs is a challenging task in SHINERS techniques. Therefore, microfabrication techniques or top-down approaches can be employed for the large-scale synthesis of pinhole-free SHINs, which have great opportunities in routine applications.132
For practical applications of SERS, two points should be emphasized: (1) the development of a novel but robust strategy for constructing SERS substrates with uniformity on the μm scale, considering that the size of the laser spot used for sampling is around 100 μm in commercial portable or handheld Raman instruments and (2) the replacement of Au- or Ag-based SERS-active substrates using other cheaper plasmonic materials to control costs to extend the SERS technique from the laboratory to industry and finally into society. For example, an Al133 substrate displayed acceptable SERS activity at the same level as Au and Ag nanostructures, whereas a great effort is required to obtain an SERS substrate with high stability and reproducibility.
To improve the sensitivity of detection, signal amplifiers such as metal nanoparticles, metallic tips, and SHINs should be designed rationally. Noble metal nanoparticles possess several fascinating features including enhanced light absorption, localized surface plasmon resonance, and Fano resonance.53,134 Remarkably, in 2008 Brevet et al. demonstrated the presence of Fano resonance in a purely plasmonic system composed of gold and silver nanoparticles where the spectrally localized surface plasmon resonance of the silver nanoparticles was coupled to the interband transitions of gold.135
The Fano resonance of noble metal nanostructures has become a ubiquitous research topic because of its high sensitivity to changes in the dielectric environment, which is a prominent feature that is essential for ultrasensitive or single-molecule sensing.40,136 Significantly, Halas et al. examined the near-field properties of individual Fano-resonant plasmonic clusters using SERS (Fig. 8).137 Recently, the same group achieved the detection of a single molecule with a Fano-resonant plasmonic tetramer substrate by surface-enhanced coherent anti-Stokes Raman scattering (SECARS).138 Therefore, well-ordered nanostructures should be fabricated that will support Fano resonance or double resonance, which will be useful for improving the detection sensitivity of SERS/SHINERS by increasing the maximum local electromagnetic field and the transmission efficiency of the scattered field.139,140
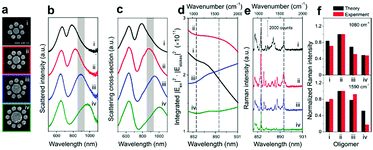 | ||
| Fig. 8 (a) SEM images of individual Au oligomer clusters: (i) octamer, (ii) nonamer, (iii) decamer, and (iv) undecamer. (b) Experimentally obtained dark-field scattering spectra. (c) Theoretical scattering spectra (FDTD). (d) Calculated enhancements in the intensity of the Stokes lines in the range of 844–931 nm (indicated by the shaded regions in (b) and (c), which correspond to 900–2000 cm−1) obtained by integrating the SERS maps of each structure over all surfaces. (e) SERS spectra of a monolayer of p-mercaptoaniline (p-MA, or p-aminothiophenol) molecules on individual clusters (i–iv) obtained using horizontally polarized pump light. The black dashed lines in (d) and (e) indicate the Stokes lines of p-MA molecules at 1080 and 1590 cm−1, respectively. (f) Comparison of the theoretical (black) and experimental (red) normalized Raman intensities of the Stokes lines at 1080 and 1590 cm−1 from all the oligomer clusters (i–iv). Reproduced from ref. 137 with permission from the American Chemical Society. | ||
3.3 Developments for better spatial and temporal resolution
An in situ Raman study is a challenging task because of the weak signals from monolayer or sub-monolayer levels of adsorbates on metal nanostructures. However, in situ SERS studies with high temporal resolution will be essential for unraveling complex reaction mechanisms and providing additional information about molecular structures and surface interactions. For instance, Ren et al. reported the use of transient electrochemical surface-enhanced Raman spectroscopy (TEC-SERS) to monitor the structural evolution of surface species at a temporal resolution that equalled those of transient electrochemical methods (e.g., cyclic voltammetry and chronoamperometry).141 TEC-SERS with a temporal resolution shorter than the charging time of the double-layer capacitance has great potential for the investigation of both reversible and irreversible electrochemical processes.To incorporate ultrafast temporal resolution into Raman studies, pump–probe methods or ultrafast spectroscopic techniques can be combined with SERS/TERS/SHINERS to increase the lifetimes of intermediate species, so that these short-lived species can be detected with greater sensitivity. For instance, Van Duyne et al. combined SERS and femtosecond stimulated Raman spectroscopy (FSRS) to investigate the dynamics of plasmonic materials, as well as investigating the contributions of environmental heterogeneities by studying more homogeneous molecular subsets.142 Developments in ultrafast SERS have enabled us to examine a wide variety of systems on ultrafast time scales, such as the time scale of molecular motion.143 Remarkably, Van Duyne et al. successfully combined picosecond-pulsed irradiation with an ultrahigh vacuum-TERS (UV-TERS) instrument.144 In this case, TERS provided correlated chemical and topographic information with higher temporal resolution.
Fig. 9 shows a schematic illustration of a pump–probe method used for Raman spectroscopy. In general, intermediate species (M) have small cross-sections and short lifetimes, and it is thus difficult to detect them by SERS. To detect intermediate species, it is necessary to utilize pump–probe methods to excite the intermediate species (M) to their excited state (M′). The excited species (M′) will have larger cross-sections and longer lifetimes, so that they can easily be detected by SERS. In general, to obtain complete information about a target system, it is necessary to detect weak adsorbates and intermediate species on target surfaces with higher temporal resolution.
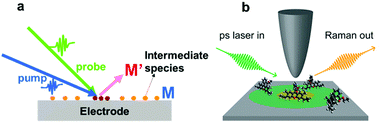 | ||
| Fig. 9 (a) Schematic illustration of pump–probe method used to detect an intermediate species (M) on an electrode surface. The excited species (M′) will have a longer lifetime and larger cross-section, which facilitates the sensitive detection of the intermediate species. (b) Schematic illustration of the experimental setup of UV-TERS with picosecond laser excitation. Reproduced from ref. 144 with permission from the American Chemical Society. | ||
In the case of time-dependent Raman measurements, high-power lasers can damage target molecules and target surfaces, and in some cases photoreactions may occur within certain molecules with the aid of metal nanoparticles.58 Thus, it is necessary to maintain an optimal laser power and exposure time to obtain relevant information about target molecules. In addition, stable and reproducible SERS substrates are preferred for time-dependent examinations. Notably, SHINs can be employed for time-dependent SERS studies to provide valuable information about intermediate species on various surfaces. SHINs exhibit significant stability in electrochemical environments and provide in situ information about electrochemical species. For instance, Zhou et al. used SHINs to examine the cathode surface in aprotic Li–O2 batteries.145 To enhance Raman signals, the authors utilized SHINs and found that the addition of water opens up a new reaction pathway for aprotic Li–O2 batteries and offers insight into the construction of catalyst-free Li–O2 batteries, which paves the way for practical applications.
Importantly, imaging modalities can be combined with Raman spectroscopy to provide physical and chemical information with high spatial and spectral resolution. The combination of imaging techniques with Raman spectroscopy can pave the way to a better understanding of the mechanisms of SERS. For instance, Hou et al. visualized single molecules with a spatial resolution of less than 1 nm using Raman imaging and scanning tunnelling microscopy.86 Thus, TERS imaging with high spatial resolution will provide new insights into electrochemical, as well as photochemical, processes at a single-molecule level.94
Rez et al. combined IR spectroscopy with electron microscopy to enable a damage-free study of biological samples.146 This technique paves the way to damage-free compositional analyses of organic functional groups at a spatial resolution of ∼10 nm that are simultaneously combined with imaging by electron microscopy. Continual advances in Raman instrumentation, together with other techniques such as imaging devices, microfluidic platforms, fluorescence spectroscopy, FT-IR spectroscopy, and pump–probe methods, will eliminate the bottlenecks in SERS and deliver a variety of information with ultrahigh sensitivity.
3.4 Combination of experimental and theoretical approaches
SERS is a multidisciplinary phenomenon, because SERS enhancement not only relies on the morphologies of nanostructures39 but also is associated with three different branches of science, namely nanoplasmonics (nanostructures and plasmons), molecular spectroscopy (photons and molecules) and surface science (molecules and nanostructures), as shown in Fig. 10. The difficulty of interpreting these three interactions in a unified manner makes the analysis of SERS data complicated, and one should be careful not to draw wrong conclusions. Importantly, experimentalists should collaborate with theoreticians to acquire detailed information about the mechanisms of enhancement, as well as detection strategies.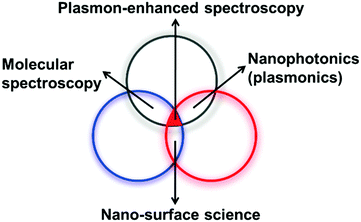 | ||
| Fig. 10 Plasmon-enhanced spectroscopy is associated with three different fields, namely, molecular spectroscopy, nanosurface science, and nanoplasmonics. | ||
For fundamental research, computational methods will be a promising tool for simulating the effect of electromagnetic radiation on the geometry of metal nanostructures and for the design of optimal geometries for increasing the overall enhancement and sensitivity of an SERS method.147 For instance, Zou and Schatz used electrodynamics calculations and reported that an enormous EM enhancement might be possible for one-dimensional arrays of nanoparticles owing to long-range photonic interactions.148 It is also worth mentioning that the dielectric constant of metal nanostructures varies with their size and nature. When the excitation wavelength is ∼650 nm, the optimum nanoparticle size for the greatest SERS enhancement is 190 and 60 nm for Ag and Au, respectively.149 Therefore, it is necessary to utilize computational tools to determine experimental parameters such as the excitation wavelength, size and shape of nanomaterials, and distances. The development of new theories and simulations is also essential for the fabrication of novel nanostructures for SERS/SHINERS/TERS, which will enrich our fundamental understanding and facilitate practical research.
For practical research, correlation spectroscopy and theoretical methods are considered to be important tools. As we discussed earlier, the detection of weakly adsorbed molecules or the identification of real constituents is a challenging task for SERS techniques. Until now, only a limited number of researchers have utilized theoretical methods such as 2D correlation spectroscopy,150 density functional theory (DFT),43 and principal component analysis151 to elucidate information about the constituents and intermediate species in complex target systems, which is not possible with normal spectral analysis. DFT calculations can also be used to calculate the adsorption energy of electrochemical species to determine the entire reaction mechanism.43,44 Moreover, DFT simulations can be performed to interpret the Raman spectra of unknown chemical species that are physically or chemically adsorbed on the surfaces of SERS-active substrates. 2D or 3D correlation spectroscopy can be used to study a complex system by resolving overlapping bands and providing information about sequences of changes in peaks.152 Thus, theoretical methods can be used to elicit valuable information about complex target systems.
To elucidate surface phenomena, SERS is used as an in situ spectroscopic technique for the identification of surface species and even surface active morphologies under experimental conditions. Thus, comprehensive simulations of the Raman spectra of surface species with their possible structures and configurations are required to support the identification of processes and cross-correlate SERS measurements with those from other methods.
To identify surface species, the experimental conditions should be included in simulations of Raman spectra in terms of the frequency and intensity.65 In the oxidation of single-crystal Pt electrodes,153 reduction of water154,155 in aqueous electrolytes, reduction of benzyl chloride on Ag electrodes in non-aqueous electrolytes, and adsorption of small molecules156 and bipyridine in ambient TERS,157 a charged surface or dipole field is used to simulate the electrode with an electrochemical potential.42 To identify surface active sites on a nanocatalyst, a roughened surface model is used to simulate the local structure. For instance, the frequency and intensity of the signals of CO adsorbed on a “mushroom” structure simulated by DFT closely correspond with SERS measurements on an Au@Pd@Pt nanoelectrocatalyst, which reveals the active sites for the formation and adsorption of CO.158,159
In addition to the simulation of SERS spectra of surface species under experimental conditions, the structures of surface species or surface active sites identified by simulations and SERS measurements should also be consistent with other experimental measurements. Because SERS signals are acquired from a minute area of a conventional sample, care should be taken in the representation of SERS. The most straightforward aspect comprises energy. According to the simulation models used for SERS spectra, energy changes or electron transfer caused by the transformation of surface species can be predicted by simulations.44 Within the theories of thermodynamics and kinetics, the aspect of energy can be directly clarified by simulations, which provide more confidence in, and information on, the conclusions obtained from SERS measurements.
3.5 Quantitative analysis
The quantitative analysis of complex samples is another challenging, albeit imperative, task, in particular in analytical chemistry. However, irreproducible metal nanostructures and SERS signals complicate the quantitative analysis of target samples, besides systematic factors such as variations in laser properties and optical alignment. For an extended overview of quantitative analysis by SERS, we refer readers to the tutorial review by Bell et al.160Metal colloids and solid substrates are susceptible to aggregation, contamination and degradation when they are exposed to ambient environments and electromagnetic radiation. For the past few decades, a burst of research activity has been seen in the preparation of metal nanostructures to circumvent the drawbacks of SERS substrates in quantitative analysis. For instance, Lipkowski et al. utilized SHINERS for the quantitative analysis of temporal changes in the passive layer on a gold electrode surface in a thiosulfate solution.161 The ν(CH2) bands of (3-aminopropyl) triethoxysilane (APTES) can be used as an internal standard to compensate for fluctuations in the surface enhancement of the electric field of the photon.
Recently, Ren et al. employed core–shell nanoparticles with embedded internal standards for reliable quantitative analysis.162 Importantly, the signal of the embedded internal standard provides effective feedback for reducing and correcting fluctuations in samples and measurement conditions, which enables label-free and reliable quantitative analysis by SERS. It should be emphasized that the internal standard molecule must be screened in advance to avoid possible overlaps of characteristic Raman peaks with those of the target molecule. Furthermore, internal standards do not solve the problems of the non-specific adsorption of molecules, signal poisoning (saturation of the SERS signal due to an excessive number of molecules) and low dynamic range.
Significantly, a few strategies such as microfluidic networks, core–shell nanoparticles (e.g., SHINs), reproducible lithographic nanostructures, and monodisperse metal nanoparticles with internal standards can deliver more reproducible results in quantitative analysis and prevent the non-specific adsorption of molecules from the solution to enable the detection by SERS of surface molecules. It is worth noting that the wavelength of the excitation laser should match those of the SERS substrates to avoid unnecessary reactions and products. In addition, SERS studies can be combined with modern multivariate data analysis to differentiate the contributions of individual components and detect subtle variations in an entire set of spectra.
3.6 Developments in Raman instrumentation
To improve the sensitivity of Raman instrumentation, efforts should be made to obtain high-resolution spectra during Raman measurements. To achieve high throughput, Raman instruments should have a limited number of dispersive or interfering elements. Previously, conventional Raman instruments employed double or triple monochromators to filter out elastically scattered laser radiation. Currently, modern Raman spectrometers employ notch filters and single-grating systems to improve signal throughput. In the near future, the detector configuration should be modified to maximize the efficiency of signal collection and transmission to achieve sensitive detection with an advanced detector. For instance, Bao et al. rationally developed a microspectrometer with a two-dimensional absorptive filter array composed of colloidal quantum dots (CQDs).163 In general, current spectrometers employ interference filters and optics, which significantly affect the photon efficiency and spectral resolution.164 Interestingly, the CQD spectrometer was based on the wavelength multiplexing principle to analyse light from a source with a wide angular distribution while maintaining the spectral resolution.Sakamoto et al. designed a portable Raman spectrometer with liquid crystal tunable filters (LCTFs) as dispersive elements.165 Specifically, LCTFs can have working apertures with sizes of up to 35 mm that utilize electronically controlled liquid crystal elements to allow the passage of selected wavelengths of light and exclude others.
Huo et al. demonstrated surface plasmon-coupled directionally enhanced Raman scattering by means of the reverse Kretschmann configuration to improve the signal collection efficiency of Raman instruments.166 Meng et al. introduced a lightweight Raman microscope that used time-correlated photon-counting detection for field and space applications.167 Thus, Raman spectrometers can be designed with a minimal number of dispersive elements or objectives with higher numerical apertures to maximize signal throughput during data collection.
Inevitably, miniaturization will decrease the energetic resolution owing to reductions in optical alignment and sensitivity, and there is also a worse signal-to-noise ratio, which mainly originates from temperature fluctuations in CCD detection. In general, the acquisition of signals from a target is the preliminary step for any instrument. Therefore, priority should be given to sensitivity over resolution for either fundamental or practical research. Most current portable Raman instruments employ such a strategy to detect target molecules by sacrificing part of the resolution. It should be noted that the optical alignment of most current portable or handheld Raman instruments is inherited from the traditional design. Thus, novel optical designs may be proposed and obtained by new manufacturing techniques, including MEMS and nanofabrication. We optimistically believe that rapid developments in instrumentation technology may improve Raman instruments and enable the introduction of new Raman instruments with fewer dispersive elements for ultrasensitive measurements. In summary, Raman instruments should be portable and able to provide high-quality Raman signals with high resolution for practical applications.
3.7 Breakthrough to market
From the above discussion, it could be concluded that after 40 years of the development of SERS techniques in both fundamental and practical directions, the issues with material, morphological and molecular generalities (see Fig. 1) were partly solved by different strategies. Molecular generality could be attained by selective sensitivity with the aid of specific capture, derivatization and labelling, etc., whereas morphological and material generalities could be achieved by TERS and SHINERS. Furthermore, with improvements in the rational physical and chemical design of nanostructured SERS substrates and the invention of easy-to-handle portable Raman facilities (the three indispensable features for commercialization, as shown in Fig. 1), we believe that now is the time for SERS techniques to achieve a breakthrough towards practical applications and commercialization.To attain commercialization, SERS has to face many competitive techniques. Because of their sensitivity, selectivity and high throughput, GC/LC-MS chromatographic methods are considered to be well-established analytical techniques in the laboratory and on the market. They seem to have unbeatable advantages over SERS for trace detection. Therefore, it is necessary to devise a way to market SERS.
In recent decades, portable and handheld Raman instruments were commercialized, which opened new horizons for the use of Raman spectroscopy in on-line or on-site detection facilities, and these have been widely employed for quality control in the pharmaceutical industry, although the spectral resolution decreased from ca. 1 cm−1 to 8–15 cm−1. In particular, the development of spatially offset Raman spectroscopy (SORS) enabled the detection and identification of concealed substances without opening packages to minimize possible exposure to harmful agents, which makes portable Raman instruments a powerful clearance tool for customs.168–170 The combination of the deep penetration properties of SORS with the high sensitivity and specificity of SERS to give the technique termed SESORS has enabled the applicability of SERS to be extended to the subsurface analysis of tissue in medicine and other diffusely scattering media to a depth of up to 45–50 mm with the aid of lab-built Raman facilities.170–172 It has been demonstrated that portable Raman instruments integrated with SERS substrates can quickly achieve on-site trace detection in various environments such as food safety, environmental monitoring and public security, which are fields where the use of traditional lab equipment is impractical.
Thus far, the practical applications of SERS have multiplied, either by micro-Raman or portable Raman facilities. Almost every report has claimed that the newly developed SERS detection method is fast, highly sensitive, highly selective, stable and easy to operate. Nevertheless, most of these methods are difficult to implement in field analysis owing to the following five issues.
(1) Spiked sample pretreatment. In practical applications, sample pretreatment is an indispensable step for trace analysis in a complicated matrix,147 whereas little or no extraction is needed, depending on the nature of the target molecules and the complexity of the sample matrix in some cases. One must take advantage of the fingerprint characteristics of SERS to shorten the pretreatment procedure. It should be emphasized that SERS detection is favourable in aqueous environments. Therefore, the pretreatment process could not simply adopt or modify the process used for GC/LC-MS, where an organic solution is used for detection. In some studies, the authors claimed that a free or simple pretreatment procedure was used for SERS analysis.173 The first point is to what extent does the spiked sample differ from the real situation. Obviously, it is not suitable to use fruits that were sprayed with pesticides for minutes, as this made the sample pretreatment much easier because the pesticides remained on the surface instead of penetrating into the fruit. Therefore, related claims should be further checked.
(2) Time required for a SERS detection procedure. SERS detection should be achieved rapidly and easily, which constitutes its advantage over GC/LC-MS. To concentrate target species or strengthen the interaction between the target and nanostructures, some researchers deposited a pure sample on the SERS substrate to let it dry freely or incubated it for a certain time to perform SERS measurements.174 The time required is difficult to control and too long, although the detection sensitivity is high.
(3) Versatility of the proposed protocol. SERS would lose its fingerprint characteristics and advantages over traditional techniques if one protocol could only detect one target, which is unfortunately the current situation with most studies in this field.
(4) Stability of SERS substrates. It is uncertain whether the stability of SERS substrates is high enough to meet the needs of commercial applications.
(5) Uniformity vs. sensitivity. It has been pointed out above that a heterogeneous substrate is typically necessary to provide ultrahigh sensitivity for the detection of single molecules, whereas a uniform substrate is required to obtain a homogeneous signal enhancement. It is difficult to satisfy both factors with one SERS substrate, and a suitable substrate has to be chosen according to the demands of different applications. For instance, for the detection of illegal additives in food, drugs of abuse and explosive materials, the higher is the sensitivity, the better is the detection, as sensitivity is preferred over homogeneous enhancement, because false negatives should be avoided as far as possible. However, in cases where quantitative analysis is highly in demand, such as bacteria in the clinical field and organic pollutants in wastewater, homogeneous substrate-to-substrate and spot-to-spot signal enhancement is the primary consideration, provided that the substrate is able to selectively detect and measure the target, which constitutes the highest criterion for the practical application of SERS in both qualitative and quantitative analysis.
Recently, with portable Raman instruments, our group has successfully achieved the trace detection of various harmful ingredients, such as illegal additives or pesticides in foods and chemical ingredients in herbal medicines, in a rapid, on-site qualitative and quantitative manner. The entire detection process, including the sample pretreatment step, could be completed in less than 15 min for both spiked and real samples using commercial substrates of which the shelf life was almost 2 years. Furthermore, the same detection protocol has the ability to detect a group of targets.175,176
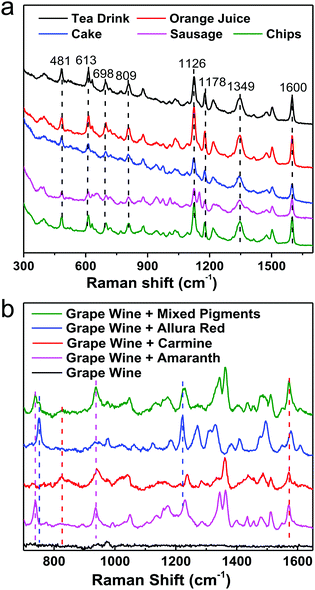
For instance, pigments in food samples can be classified into three types: acidic (carmine, etc.), neutral (Sudan red, etc.) and basic dyes (auramine, etc.), considering the similarities and differences in their chemical structures. By integrating the same simple pretreatment procedure, we developed a non-targeting, qualitative, and highly sensitive protocol for the detection of various acidic dyes in different foods (milk, meat and cake, etc.), which could be performed in 15 min, as shown in Fig. 11a. It should be emphasized that the matrices are different in these foods, for example, high concentrations of tea polyphenols, carotene, vegetable fats, animal fats and starch in tea, juice, cake, sausage and chips, respectively. Nevertheless, the same optimized pretreatment step eliminated interference from the matrix efficiently, whereby the SERS spectra that were obtained were fairly similar and the differences in Raman intensity between different samples were due to interference from matrix residues in different foods. Furthermore, we successfully distinguished three acidic dyes (carmine, allura red and amaranth), which are illegal additives, in grape wine simultaneously in 1 min without any pretreatment step, as shown in Fig. 11b. However, for commercial applications pretreatment is highly recommended because it will eliminate most matrix interference from different foods, which will enable SERS detection protocols to be carried out with one kit. Although some exciting progress has been achieved, it could be a long time until SERS has been developed into a general tool for trace detection in complicated target systems in daily life and society.
Conclusions
By reviewing the historical developments and key limitations associated with SERS, we have discussed various bottlenecks and breakthroughs in SERS in the past four decades with relevant examples. We have attempted to enumerate the obstacles to the future development of SERS and members of the same family of techniques. We have also pointed out possible strategies for further improving the sensitivity and versatility of SERS in both fundamental and practical research. Optimistically, we envisage that nanostructure-based Raman techniques, together with rapid developments in nanoscience, will undoubtedly reveal new phenomena and may open up new avenues in the direction of fundamental research. In the other direction, namely, applications and commercialization, by the continued development of methods and instrumentation future efforts will provide next-generation plasmonic substrates with significant sensitivity, reproducibility, and stability to deliver meaningful applications in the whole scientific community. SERS techniques will also emerge from laboratories to enter the homes of ordinary people in the form of reliable, cost-effective, and easy-to-handle instruments for routine usage in real-world environments.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work has been made possible by the continuous financial support of the Natural Science Foundation of China (91427304, 21533006, 21321062, 21522508, 20021002 and 21473140) and the Ministry of Science and Technology (2015CB932300). Whenever work from the authors’ group is mentioned, we are very grateful for the contribution of the self-motivated and hard-working students and all other group members.Notes and references
- C. V. Raman and K. S. Krishnan, Nature, 1928, 121, 501–502 CrossRef CAS.
- J. R. Baena and B. Lendl, Curr. Opin. Chem. Biol., 2004, 8, 534–539 CrossRef CAS PubMed.
- A. Kudelski, Talanta, 2008, 76, 1–8 CrossRef CAS PubMed.
- E. Smith and G. Dent, Modern Raman Spectroscopy – A Practical Approach, John Wiley & Sons, Ltd, New York, 2005 Search PubMed.
- R. L. McCreery, Raman Spectroscopy for Chemical Analysis, John Wiley & Sons, Ltd, New York, 2005 Search PubMed.
- P. J. Hendra, I. D. M. Turner, E. J. Loader and M. Stacey, J. Phys. Chem., 1974, 78, 300–304 CrossRef CAS.
- M. Fleischmann, P. J. Hendra and A. J. McQuillan, Chem. Phys. Lett., 1974, 26, 163–166 CrossRef CAS.
- D. L. Jeanmaire and R. P. Van Duyne, J. Electroanal. Chem. Interfacial Electrochem., 1977, 84, 1–20 CrossRef CAS.
- M. G. Albrecht and J. A. Creighton, J. Am. Chem. Soc., 1977, 99, 5215–5217 CrossRef CAS.
- M. Moskovits, J. Chem. Phys., 1978, 69, 4159–4161 CrossRef CAS.
- J. A. Creighton, C. G. Blatchford and M. G. Albrecht, J. Chem. Soc., Faraday Trans. 2, 1979, 75, 790–798 RSC.
- M. Faraday, Experimental Relations of Gold (and other Metals) to Light, Philos. Trans. R. Soc. London, 1857, 147, 145–181 CrossRef.
- R. P. Van Duyne, in Chemical and Biochemical Applications of Lasers, ed. C. B. Moore, Academic Press, Amsterdam, 1979 Search PubMed.
- P. L. Stiles, J. A. Dieringer, N. C. Shah and R. P. Van Duyne, Annu. Rev. Anal. Chem., 2008, 1, 601–626 CrossRef CAS PubMed.
- S. Schlücker, Angew. Chem., Int. Ed., 2014, 53, 4756–4795 CrossRef PubMed.
- B. Sharma, R. R. Frontiera, A. I. Henry, E. Ringe and R. P. Van Duyne, Mater. Today, 2012, 15, 16–25 CrossRef CAS.
- M. Moskovits, J. Raman Spectrosc., 2005, 36, 485–496 CrossRef CAS.
- E. J. Zeman and G. C. Schatz, J. Phys. Chem., 1987, 91, 634–643 CrossRef CAS.
- A. Campion and P. Kambhampati, Chem. Soc. Rev., 1998, 27, 241–250 RSC.
- Y. S. Yamamoto, Y. Ozaki and T. Itoh, J. Photochem. Photobiol., C, 2014, 21, 81–104 CrossRef CAS.
- K. Kneipp, H. Kneipp, I. Itzkan, R. R. Dasari and M. S. Feld, Chem. Rev., 1999, 99, 2957–2976 CrossRef CAS PubMed.
- Z. Q. Tian, J. Raman Spectrosc., 2005, 36, 466–470 CrossRef CAS.
- D. Graham and R. Goodacre, Chem. Soc. Rev., 2008, 37, 883–884 RSC.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442–453 CrossRef CAS PubMed.
- C. Mavroyannis, Chem. Phys. Lett., 1978, 56, 263–266 CrossRef CAS.
- M. Moskovits, Chem. Phys. Lett., 1983, 98, 498–502 CrossRef CAS.
- R. Aroca, Surface-Enhanced Vibrational Spectroscopy, John Wiley & Sons, Ltd, New York, 2007 Search PubMed.
- M. Kerker, Selected Papers on Surface Enhanced Raman Scattering, SPIE Milestone Series, Bellingham, 1990 Search PubMed.
- M. Kerker, Acc. Chem. Res., 1984, 17, 271–277 CrossRef CAS.
- E. C. Le Ru and P. G. Etchegoin, Annu. Rev. Phys. Chem., 2012, 63, 65–87 CrossRef CAS PubMed.
- H. Metiu, Prog. Surf. Sci., 1984, 17, 153–320 CrossRef CAS.
- H. Metiu and P. Das, Annu. Rev. Phys. Chem., 1984, 35, 507–536 CrossRef CAS.
- M. Moskovits, Rev. Mod. Phys., 1985, 57, 783–826 CrossRef CAS.
- M. Moskovits, J. Chem. Phys., 1982, 77, 4408–4416 CrossRef CAS.
- A. Otto, I. Mrozek, H. Grabhorn and W. Akemann, J. Phys.: Condens. Matter, 1992, 4, 1143–1212 CrossRef CAS.
- G. C. Schatz, Acc. Chem. Res., 1984, 17, 370–376 CrossRef CAS.
- L. Jensen, C. M. Aikens and G. C. Schatz, Chem. Soc. Rev., 2008, 37, 1061–1073 RSC.
- W. E. Smith, Chem. Soc. Rev., 2008, 37, 955–964 RSC.
- S. Y. Ding, X. M. Zhang, B. Ren and Z. Q. Tian, Encyclopedia of Analytical Chemistry, John Wiley & Sons, Ltd, New York, 2013 Search PubMed.
- S. Y. Ding, E. M. You, Z. Q. Tian and M. Moskovits, Chem. Soc. Rev., 2017, 46, 4042–4076 RSC.
- C. L. Haynes, A. D. McFarland and R. P. Van Duyne, Anal. Chem., 2005, 77, 338–346 CrossRef.
- S. L. Kleinman, R. R. Frontiera, A. I. Henry, J. A. Dieringer and R. P. Van Duyne, Phys. Chem. Chem. Phys., 2013, 15, 21–36 RSC.
- A. Wang, Y. F. Huang, U. K. Sur, D. Y. Wu, B. Ren, S. Rondinini, C. Amatore and Z. Q. Tian, J. Am. Chem. Soc., 2010, 132, 9534–9536 CrossRef CAS PubMed.
- Y. F. Huang, D. Y. Wu, A. Wang, B. Ren, S. Rondinini, Z. Q. Tian and C. Amatore, J. Am. Chem. Soc., 2010, 132, 17199–17210 CrossRef CAS PubMed.
- J. H. Tian, B. Liu, X. Li, Z. L. Yang, B. Ren, S. T. Wu, N. J. Tao and Z. Q. Tian, J. Am. Chem. Soc., 2006, 128, 14748–14749 CrossRef CAS PubMed.
- D. R. Ward, N. J. Halas, J. W. Ciszek, J. M. Tour, Y. Wu, P. Nordlander and D. Natelson, Nano Lett., 2008, 8, 919–924 CrossRef CAS PubMed.
- T. Konishi, M. Kiguchi, M. Takase, F. Nagasawa, H. Nabika, K. Ikeda, K. Uosaki, K. Ueno, H. Misawa and K. Murakoshi, J. Am. Chem. Soc., 2013, 135, 1009–1014 CrossRef CAS PubMed.
- M. F. Cardinal, E. Vander Ende, R. A. Hackler, M. O. McAnally, P. C. Stair, G. C. Schatz and R. P. Van Duyne, Chem. Soc. Rev., 2017, 46, 3886–3903 RSC.
- D. Cialla-May, X. S. Zheng, K. Weber and J. Popp, Chem. Soc. Rev., 2017, 46, 3945–3961 RSC.
- D. Graham, M. Moskovits and Z. Q. Tian, Chem. Soc. Rev., 2017, 46, 3864–3865 RSC.
- S. M. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS PubMed.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667–1670 CrossRef CAS.
- J. Reguera, J. Langer, D. Jimenez de Aberasturi and L. M. Liz-Marzan, Chem. Soc. Rev., 2017, 46, 3866–3885 RSC.
- E. C. Le Ru and P. G. Etchegoin, in Principles of Surface-Enhanced Raman Spectroscopy, ed. E. C. L. Ru and P. G. Etchegoin, Elsevier, Amsterdam, 2009 Search PubMed.
- Z. Q. Tian, B. Ren and D. Y. Wu, J. Phys. Chem. B, 2002, 106, 9463–9483 CrossRef CAS.
- M. Moskovits, L. L. Tay, J. Yang and T. Haslett, in Optical Properties of Nanostructured Random Media, ed. V. M. Shalaev, Springer-Verlag Berlin, Berlin, 2002 Search PubMed.
- J. F. Li, J. R. Anema, T. Wandlowski and Z. Q. Tian, Chem. Soc. Rev., 2015, 44, 8399–8409 RSC.
- Y. F. Huang, H. P. Zhu, G. K. Liu, D. Y. Wu, B. Ren and Z. Q. Tian, J. Am. Chem. Soc., 2010, 132, 9244–9246 CrossRef CAS PubMed.
- A. Bruckbauer and A. Otto, J. Raman Spectrosc., 1998, 29, 665–672 CrossRef CAS.
- Y. X. Chen and A. Otto, J. Raman Spectrosc., 2005, 36, 736–747 CrossRef CAS.
- M. J. Natan, Faraday Discuss., 2006, 132, 321–328 RSC.
- R. J. C. Brown and M. J. T. Milton, J. Raman Spectrosc., 2008, 39, 1313–1326 CrossRef CAS.
- Y. Fang, N. H. Seong and D. D. Dlott, Science, 2008, 321, 388–392 CrossRef CAS PubMed.
- R. P. Van Duyne and J. P. Haushalter, J. Phys. Chem., 1983, 87, 2999–3003 CrossRef CAS.
- D. Y. Wu, J. F. Li, B. Ren and Z. Q. Tian, Chem. Soc. Rev., 2008, 37, 1025–1041 RSC.
- X. D. Lin, J. F. Li, Y. F. Huang, X. D. Tian, V. Uzayisenga, S. B. Li, B. Ren and Z. Q. Tian, J. Electroanal. Chem., 2013, 688, 5–11 CrossRef CAS.
- L. W. H. Leung and M. J. Weaver, J. Am. Chem. Soc., 1987, 109, 5113–5119 CrossRef CAS.
- G. Mengoli, M. M. Musiani, M. Fleischmann, B. Mao and Z. Q. Tian, Electrochim. Acta, 1987, 32, 1239–1245 CrossRef CAS.
- Z. Q. Tian, B. Ren, J. F. Li and Z. L. Yang, Chem. Commun., 2007, 3514–3534 RSC.
- B. Mir-Simon, I. Reche-Perez, L. Guerrini, N. Pazos-Perez and R. A. Alvarez-Puebla, Chem. Mater., 2015, 27, 950–958 CrossRef CAS.
- J. L. Gong, J. H. Jiang, H. F. Yang, G. L. Shen, R. Q. Yu and Y. Ozaki, Anal. Chim. Acta, 2006, 564, 151–157 CrossRef CAS.
- A. F. McCabe, C. Eliasson, R. A. Prasath, A. Hernandez-Santana, L. Stevenson, I. Apple, P. A. G. Cormack, D. Graham, W. E. Smith, P. Corish, S. J. Lipscomb, E. R. Holland and P. D. Prince, Faraday Discuss., 2006, 132, 303–308 RSC.
- X. J. Chen, G. Cabello, D. Y. Wu and Z. Q. Tian, J. Photochem. Photobiol., C, 2014, 21, 54–80 CrossRef CAS.
- S. Y. Ding, J. Yi, J. F. Li, B. Ren, D. Y. Wu, R. Panneerselvam and Z. Q. Tian, Nat. Rev. Mater., 2016, 1, 16021 CrossRef CAS.
- J. F. Li, Y. J. Zhang, S. Y. Ding, R. Panneerselvam and Z. Q. Tian, Chem. Rev., 2017, 117, 5002–5069 CrossRef CAS PubMed.
- J. Wessel, J. Opt. Soc. Am. B, 1985, 2, 1538–1541 CrossRef CAS.
- R. M. Stockle, Y. D. Suh, V. Deckert and R. Zenobi, Chem. Phys. Lett., 2000, 318, 131–136 CrossRef CAS.
- M. S. Anderson, Appl. Phys. Lett., 2000, 76, 3130–3132 CrossRef CAS.
- B. Pettinger, G. Picardi, R. Schuster and G. Ertl, Electrochemistry, 2000, 68, 942–949 CAS.
- N. Hayazawa, Y. Inouye, Z. Sekkat and S. Kawata, Opt. Commun., 2000, 183, 333–336 CrossRef CAS.
- T. Deckert-Gaudig, A. Taguchi, S. Kawata and V. Deckert, Chem. Soc. Rev., 2017, 46, 4077–4110 RSC.
- M. Richard-Lacroix, Y. Zhang, Z. Dong and V. Deckert, Chem. Soc. Rev., 2017, 46, 3922–3944 RSC.
- P. Verma, Chem. Rev., 2017, 117, 6447–6466 CrossRef CAS PubMed.
- M. D. Sonntag, E. A. Pozzi, N. Jiang, M. C. Hersam and R. P. Van Duyne, J. Phys. Chem. Lett., 2014, 5, 3125–3130 CrossRef CAS PubMed.
- M. D. Sonntag, D. Chulhai, T. Seideman, L. Jensen and R. P. Van Duyne, J. Am. Chem. Soc., 2013, 135, 17187–17192 CrossRef CAS PubMed.
- C. Chen, N. Hayazawa and S. Kawata, Nat. Commun., 2014, 5, 3312 Search PubMed.
- J. H. Zhong, X. Jin, L. Meng, X. Wang, H. S. Su, Z. L. Yang, C. T. Williams and B. Ren, Nat. Nanotechnol., 2017, 12, 132–136 CrossRef CAS PubMed.
- R. Zhang, Y. Zhang, Z. C. Dong, S. Jiang, C. Zhang, L. G. Chen, L. Zhang, Y. Liao, J. Aizpurua, Y. Luo, J. L. Yang and J. G. Hou, Nature, 2013, 498, 82–86 CrossRef CAS PubMed.
- C. Zhang, B. Q. Chen and Z. Y. Li, J. Phys. Chem. C, 2015, 119, 11858–11871 CAS.
- S. Duan, G. Tian and Y. Luo, Angew. Chem., Int. Ed., 2016, 128, 1053–1057 CrossRef.
- S. Y. Ding and Z. Q. Tian, Natl. Sci. Rev., 2014, 1, 4–5 CrossRef.
- Z. D. Schultz, J. M. Marr and H. Wang, Nanophotonics, 2014, 3, 91–104 CrossRef CAS.
- T. Deckert-Gaudig, E. Bailo and V. Deckert, Phys. Chem. Chem. Phys., 2009, 11, 7360–7362 RSC.
- T. Schmid, L. Opilik, C. Blum and R. Zenobi, Angew. Chem., Int. Ed., 2013, 52, 5940–5954 CrossRef CAS PubMed.
- G. Sharma, T. Deckert-Gaudig and V. Deckert, Adv. Drug Delivery Rev., 2015, 89, 42–56 CrossRef CAS PubMed.
- E. Bailo and V. Deckert, Angew. Chem., Int. Ed., 2008, 47, 1658–1661 CrossRef CAS PubMed.
- E. M. van Schrojenstein Lantman, T. Deckert-Gaudig, A. J. G. Mank, V. Deckert and B. M. Weckhuysen, Nat. Nanotechnol., 2012, 7, 583–586 CrossRef CAS PubMed.
- T. X. Huang, S. C. Huang, M. H. Li, Z. C. Zeng, X. Wang and B. Ren, Anal. Bioanal. Chem., 2015, 407, 8177–8195 CrossRef CAS PubMed.
- J. F. Li, Y. F. Huang, Y. Ding, Z. L. Yang, S. B. Li, X. S. Zhou, F. R. Fan, W. Zhang, Z. Y. Zhou, D. Y. Wu, B. Ren, Z. L. Wang and Z. Q. Tian, Nature, 2010, 464, 392–395 CrossRef CAS PubMed.
- J. R. Anema, J. F. Li, Z. L. Yang, B. Ren and Z. Q. Tian, Annu. Rev. Anal. Chem., 2011, 4, 129–150 CrossRef CAS PubMed.
- J. F. Li, X. D. Tian, S. B. Li, J. R. Anema, Z. L. Yang, Y. Ding, Y. F. Wu, Y. M. Zeng, Q. Z. Chen, B. Ren, Z. L. Wang and Z. Q. Tian, Nat. Protoc., 2013, 8, 52–65 CrossRef CAS PubMed.
- T. A. Galloway, L. Cabo-Fernandez, I. Aldous, F. Braga and L. Hardwick, Faraday Discuss., 2017 10.1039/C7FD00151G.
- P. P. Fang, X. Lu, H. Liu and Y. Tong, Trends Anal. Chem., 2015, 66, 103–117 CrossRef CAS.
- Y. Liu, Y. Hu and J. Zhang, J. Phys. Chem. C, 2014, 118, 8993–8998 CAS.
- X. Bian, Z. L. Song, Y. Qian, W. Gao, Z. Q. Cheng, L. Chen, H. Liang, D. Ding, X. K. Nie, Z. Chen and W. Tan, Sci. Rep., 2014, 4, 6093 CrossRef CAS PubMed.
- B. Liu, A. Blaszczyk, M. Mayor and T. Wandlowski, ACS Nano, 2011, 5, 5662–5672 CrossRef CAS PubMed.
- M. Zhang, L. J. Yu, Y. F. Huang, J. W. Yan, G. K. Liu, D. Y. Wu, Z. Q. Tian and B. W. Mao, Chem. Commun., 2014, 50, 14740–14743 RSC.
- C. Y. Li, J. C. Dong, X. Jin, S. Chen, R. Panneerselvam, A. V. Rudnev, Z. L. Yang, J. F. Li, T. Wandlowski and Z. Q. Tian, J. Am. Chem. Soc., 2015, 137, 7648–7651 CrossRef CAS PubMed.
- J. F. Li, Y. J. Zhang, A. V. Rudnev, J. R. Anema, S. B. Li, W. J. Hong, P. Rajapandiyan, J. Lipkowski, T. Wandlowski and Z. Q. Tian, J. Am. Chem. Soc., 2015, 137, 2400–2408 CrossRef CAS PubMed.
- B. Y. Wen, X. Jin, Y. Li, Y. H. Wang, C. Y. Li, M. M. Liang, R. Panneerselvam, Q. C. Xu, D. Y. Wu, Z. L. Yang, J. F. Li and Z. Q. Tian, Analyst, 2016, 141, 3731–3736 RSC.
- J. C. Dong, R. Panneerselvam, Y. Lin, X. D. Tian and J. F. Li, Adv. Opt. Mater., 2016, 4, 1144–1158 CrossRef CAS.
- H. Zhang, C. Wang, H. L. Sun, G. Fu, S. Chen, Y. J. Zhang, B. H. Chen, J. R. Anema, Z. L. Yang, J. F. Li and Z. Q. Tian, Nat. Commun., 2017, 8, 15447 CrossRef CAS PubMed.
- A. Wokaun, J. G. Bergman, J. P. Heritage, A. M. Glass, P. F. Liao and D. H. Olson, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 24, 849–856 CrossRef CAS.
- M. Osawa, Near-Field Optics and Surface Plasmon Polaritons, 2001, 81, 163–187 CAS.
- J. R. Lakowicz, C. D. Geddes, I. Gryczynski, J. Malicka, Z. Gryczynski, K. Aslan, J. Lukomska, E. Matveeva, J. A. Zhang, R. Badugu and J. Huang, J. Fluoresc., 2004, 14, 425–441 CrossRef CAS PubMed.
- W. H. Li, X. Y. Li and N. T. Yu, Chem. Phys. Lett., 2000, 327, 153–161 CrossRef CAS.
- S. Baldelli, A. S. Eppler, E. Anderson, Y. R. Shen and G. A. Somorjai, J. Chem. Phys., 2000, 113, 5432–5438 CrossRef CAS.
- X. Hua, D. V. Voronine, C. W. Ballmann, A. M. Sinyukov, A. V. Sokolov and M. O. Scully, Phys. Rev. A: At., Mol., Opt. Phys., 2014, 89, 7 CrossRef.
- T. A. Yano and S. Kawata, Frontiers of Surface-Enhanced Raman Scattering, John Wiley & Sons, Ltd, New York, 2014 Search PubMed.
- J. F. Li and Z. Q. Tian, Frontiers of Surface-Enhanced Raman Scattering, John Wiley & Sons, Ltd, New York, 2014 Search PubMed.
- Z. Q. Tian and B. Ren, Annu. Rev. Phys. Chem., 2004, 55, 197–229 CrossRef CAS PubMed.
- N. P. W. Pieczonka and R. F. Aroca, Chem. Soc. Rev., 2008, 37, 946–954 RSC.
- W. Liao and X. Lu, Trends Food Sci. Technol., 2016, 54, 103–113 CrossRef CAS.
- U. S. Dinish and M. Olivo, in Handbook of Photonics for Biomedical Engineering, ed. A. H. P. Ho, D. Kim and M. G. Somekh, Springer Netherlands, Dordrecht, 2017 Search PubMed.
- Z. C. Zeng, S. C. Huang, D. Y. Wu, L. Y. Meng, M. H. Li, T. X. Huang, J. H. Zhong, X. Wang, Z. L. Yang and B. Ren, J. Am. Chem. Soc., 2015, 137, 11928–11931 CrossRef CAS PubMed.
- D. Kurouski, M. Mattei and R. P. Van Duyne, Nano Lett., 2015, 15, 7956–7962 CrossRef CAS PubMed.
- C. C. Neacsu, S. Berweger, R. L. Olmon, L. V. Saraf, C. Ropers and M. B. Raschke, Nano Lett., 2010, 10, 592–596 CrossRef CAS PubMed.
- C. Y. Li, M. Meng, S. C. Huang, L. Li, S. R. Huang, S. Chen, L. Y. Meng, R. Panneerselvam, S. J. Zhang, B. Ren, Z. L. Yang, J. F. Li and Z. Q. Tian, J. Am. Chem. Soc., 2015, 137, 13784–13787 CrossRef CAS PubMed.
- M. Osawa, in Near-Field Optics and Surface Plasmon Polaritons, ed. S. Kawata, Springer Berlin Heidelberg, 2001 Search PubMed.
- D. Wang, L. Guo, R. Huang, B. Qiu, Z. Lin and G. Chen, Sci. Rep., 2015, 5, 7954 CrossRef CAS PubMed.
- A. R. Guerrero and R. F. Aroca, Angew. Chem., Int. Ed., 2011, 50, 665–668 CrossRef CAS PubMed.
- S. Y. Ding, E. M. You, J. Yi, J. F. Li and Z. Q. Tian, Faraday Discuss., 2017 10.1039/C7FD00144D.
- S. Tian, O. Neumann, M. J. McClain, X. Yang, L. Zhou, C. Zhang, P. Nordlander and N. J. Halas, Nano Lett., 2017, 17, 5071–5077 CrossRef CAS PubMed.
- K. Thyagarajan, J. Butet and O. J. F. Martin, Nano Lett., 2013, 13, 1847–1851 CrossRef CAS PubMed.
- G. Bachelier, I. Russier-Antoine, E. Benichou, C. Jonin, N. Del Fatti, F. Vallee and P. F. Brevet, Phys. Rev. Lett., 2008, 101, 197401 CrossRef CAS PubMed.
- B. Luk'yanchuk, N. I. Zheludev, S. A. Maier, N. J. Halas, P. Nordlander, H. Giessen and C. T. Chong, Nat. Mater., 2010, 9, 707–715 CrossRef PubMed.
- J. Ye, F. F. Wen, H. Sobhani, J. B. Lassiter, P. Van Dorpe, P. Nordlander and N. J. Halas, Nano Lett., 2012, 12, 1660–1667 CrossRef CAS PubMed.
- Y. Zhang, Y. R. Zhen, O. Neumann, J. K. Day, P. Nordlander and N. J. Halas, Nat. Commun., 2014, 5, 4424 CAS.
- N. J. Halas, S. Lal, W. S. Chang, S. Link and P. Nordlander, Chem. Rev., 2011, 111, 3913–3961 CrossRef CAS PubMed.
- M. Rahmani, B. Luk'yanchuk and M. Hong, Laser Photonics Rev., 2013, 7, 329–349 CrossRef CAS.
- C. Zong, C. J. Chen, M. Zhang, D. Y. Wu and B. Ren, J. Am. Chem. Soc., 2015, 137, 11768–11774 CrossRef CAS PubMed.
- R. R. Frontiera, A. I. Henry, N. L. Gruenke and R. P. Van Duyne, J. Phys. Chem. Lett., 2011, 2, 1199–1203 CrossRef CAS PubMed.
- N. L. Gruenke, M. F. Cardinal, M. O. McAnally, R. R. Frontiera, G. C. Schatz and R. P. Van Duyne, Chem. Soc. Rev., 2016, 45, 2263–2290 RSC.
- E. A. Pozzi, M. D. Sonntag, N. Jiang, N. Chiang, T. Seideman, M. C. Hersam and R. P. Van Duyne, J. Phys. Chem. Lett., 2014, 5, 2657–2661 CrossRef CAS PubMed.
- Y. Qiao, S. Wu, J. Yi, Y. Sun, S. Guo, S. Yang, P. He and H. Zhou, Angew. Chem., Int. Ed., 2017, 56, 4960–4964 CrossRef CAS PubMed.
- P. Rez, T. Aoki, K. March, D. Gur, O. L. Krivanek, N. Dellby, T. C. Lovejoy, S. G. Wolf and H. Cohen, Nat. Commun., 2016, 7, 10945 CrossRef CAS PubMed.
- J. Zhao, A. O. Pinchuk, J. M. McMahon, S. Z. Li, L. K. Ausman, A. L. Atkinson and G. C. Schatz, Acc. Chem. Res., 2008, 41, 1710–1720 CrossRef CAS PubMed.
- S. L. Zou and G. C. Schatz, Chem. Phys. Lett., 2005, 403, 62–67 CrossRef CAS.
- J. T. Krug, G. D. Wang, S. R. Emory and S. M. Nie, J. Am. Chem. Soc., 1999, 121, 9208–9214 CrossRef CAS.
- I. Noda and Y. Ozaki, Two-Dimensional Correlation Spectroscopy – Applications in Vibrational and Optical Spectroscopy, John Wiley & Sons, Ltd, New York, 2005 Search PubMed.
- G. Rusciano, G. Zito, R. Isticato, T. Sirec, E. Ricca, E. Bailo and A. Sasso, ACS Nano, 2014, 8, 12300–12309 CrossRef CAS PubMed.
- W. Ji, N. Spegazzini, Y. Kitahama, Y. Chen, B. Zhao and Y. Ozaki, J. Phys. Chem. Lett., 2012, 3, 3204–3209 CrossRef CAS PubMed.
- Y. F. Huang, P. J. Kooyman and M. T. M. Koper, Nat. Commun., 2016, 7, 12440 CrossRef CAS PubMed.
- J. F. Li, Y. F. Huang, S. Duan, R. Pang, D. Y. Wu, B. Ren, X. Xuand and Z. Q. Tian, Phys. Chem. Chem. Phys., 2010, 12, 2493–2502 RSC.
- S. Duan, D. Y. Wu, X. Xu, Y. Luo and Z. Q. Tian, J. Phys. Chem. C, 2010, 114, 4051–4056 CAS.
- S. A. Wasileski, M. T. M. Koper and M. J. Weaver, J. Am. Chem. Soc., 2002, 124, 2796–2805 CrossRef CAS PubMed.
- Z. Liu, S. Y. Ding, Z. B. Chen, X. Wang, J. H. Tian, J. R. Anema, X. S. Zhou, D. Y. Wu, B. W. Mao, X. Xu, B. Ren and Z. Q. Tian, Nat. Commun., 2011, 2, 305 CrossRef PubMed.
- P. P. Fang, S. Duan, X. D. Lin, J. R. Anema, J. F. Li, O. Buriez, Y. Ding, F. R. Fan, D. Y. Wu, B. Ren, Z. L. Wang, C. Amatore and Z. Q. Tian, Chem. Sci., 2011, 2, 531–539 RSC.
- S. Duan, P. P. Fang, F. R. Fan, I. Broadwell, F. Z. Yang, D. Y. Wu, B. Ren, C. Amatore, Y. Luo, X. Xu and Z. Q. Tian, Phys. Chem. Chem. Phys., 2011, 13, 5441–5449 RSC.
- S. E. J. Bell and N. M. S. Sirimuthu, Chem. Soc. Rev., 2008, 37, 1012–1024 RSC.
- S. R. Smith, J. J. Leitch, C. Zhou, J. Mirza, S. B. Li, X. D. Tian, Y. F. Huang, Z. Q. Tian, J. Y. Baron, Y. Choi and J. Lipkowski, Anal. Chem., 2015, 87, 3791–3799 CrossRef CAS PubMed.
- W. Shen, X. Lin, C. Jiang, C. Li, H. Lin, J. Huang, S. Wang, G. Liu, X. Yan, Q. Zhong and B. Ren, Angew. Chem., Int. Ed., 2015, 127, 7308–7312 CrossRef PubMed.
- J. Bao and M. G. Bawendi, Nature, 2015, 523, 67–70 CrossRef CAS PubMed.
- S. W. Wang, C. Xia, X. Chen, W. Lu, M. Li, H. Wang, W. Zheng and T. Zhang, Opt. Lett., 2007, 32, 632–634 CrossRef PubMed.
- A. Sakamoto, S. Ochiai, H. Higashiyama, K. Masutani, J. I. Kimura, E. Koseto-Horyu and M. Tasumi, J. Raman Spectrosc., 2012, 43, 787–791 CrossRef CAS.
- S. X. Huo, Q. Liu, S. H. Cao, W. P. Cai, L. Y. Meng, K. X. Xie, Y. Y. Zhai, C. Zong, Z. L. Yang, B. Ren and Y. Q. Li, J. Phys. Chem. Lett., 2015, 6, 2015–2019 CrossRef CAS PubMed.
- Z. Meng, G. I. Petrov, S. Cheng, J. A. Jo, K. K. Lehmann, V. V. Yakovlev and M. O. Scully, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 12315–12320 CrossRef CAS PubMed.
- P. Matousek, I. P. Clark, E. R. C. Draper, M. D. Morris, A. E. Goodship, N. Everall, M. Towrie, W. F. Finney and A. W. Parker, Appl. Spectrosc., 2005, 59, 393–400 CrossRef CAS PubMed.
- W. J. Olds, E. Jaatinen, P. Fredericks, B. Cletus, H. Panayiotou and E. L. Izake, Forensic Sci. Int., 2011, 212, 69–77 CrossRef CAS PubMed.
- P. Matousek and N. Stone, Chem. Soc. Rev., 2016, 45, 1794 RSC.
- N. Stone, K. Faulds, D. Graham and P. Matousek, Anal. Chem., 2010, 82, 3969 CrossRef CAS PubMed.
- N. Stone, M. Kerssens, G. R. Lloyd, K. Faulds, D. Graham and P. Matousek, Chem. Sci., 2011, 2, 776 RSC.
- W. Wijaya, S. Pang, T. P. Labuza and L. He, J. Food Sci., 2014, 79, 743–747 CrossRef PubMed.
- Z. Zhang, Q. Yu, H. Li, A. Mustapha and M. Lin, J. Food Sci., 2015, 80, 450–458 CrossRef PubMed.
- Q. Chen, Y. Zeng, H. Lin, H. Chen, Z. Tian and G. Liu, J. Xiamen Univ., Nat. Sci., 2016, 55, 754–759 Search PubMed , in Chinese.
- L. Zhang, Y. Zeng, J. Zhao, H. Chen, J. Kong, Q. Chen, H. Lin, Z. Tian and G. Liu, Sci. China: Chem., 2017, 47, 794–800 Search PubMed , in Chinese.
| This journal is © The Royal Society of Chemistry 2018 |