High magnetic relaxivity in a fluorescent CdSe/CdS/ZnS quantum dot functionalized with MRI contrast molecules†
Abstract
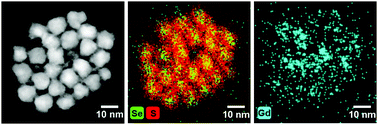
Light emitting semiconducting quantum dots show great promise as solar cells, optoelectronic devices and multimodal imaging probes. Here we demonstrate successful grafting of a thiol-functionalised GdIII MRI contrast agent onto the surface of core-multishell CdSe/CdS/ZnS quantum dots. The resulting nanoprobe exhibits intense photoluminescence and unprecedentedly large T1 relaxivity of 6800 mM−1 s−1 per nanoparticle due to secure implanting of ca. 620 magnetic centers per quantum dot unit.
- This article is part of the themed collection: Commemorating Michael Faraday (1791-1867)


 Please wait while we load your content...
Please wait while we load your content...