Open Access Article
Open Access ArticleLow-toxicity metallosomes for biomedical applications by self-assembly of organometallic metallosurfactants and phospholipids†
M.
Marín-García
 a,
N.
Benseny-Cases
a,
N.
Benseny-Cases
 b,
M.
Camacho
b,
M.
Camacho
 c,
J.
Suades
c,
J.
Suades
 *d and
R.
Barnadas-Rodríguez
*d and
R.
Barnadas-Rodríguez
 *a
*a
aBiophysics Unit/Center for Biophysical Studies, Department of Biochemistry and Molecular Biology, Faculty of Medicine, Universitat Autònoma de Barcelona, Avda. de Can Domènech, 08193 Cerdanyola, Spain. E-mail: Ramon.Barnadas@uab.cat
bALBA Synchrotron, Carrer de la Llum 2-26, 08290 Cerdanyola, Spain
cLaboratory of Angiology, Vascular Biology and Inflammation/Institute of Biomedical Research, Hospital de la Santa Creu i de Sant Pau, Universitat Autònoma de Barcelona, Sant Antoni Maria Claret, 167, 08025 Barcelona, Spain
dDepartament de Química, Edifici C, Universitat Autònoma de Barcelona, 08193 Cerdanyola, Spain. E-mail: Joan.Suades@uab.cat
First published on 29th June 2017
Abstract
A new and convenient strategy for the preparation of metallosomes has been developed by mixing organometallic metallosurfactants and phospholipids. These aggregates show the characteristic properties of liposomes (stability upon dilution and low toxicity) and the toxicity is at least ten-fold lower than that of the metallosurfactant aggregates without phospholipids.
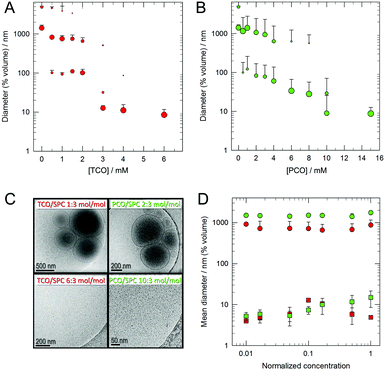
The preparation and study of nanomaterials by self-assembly in aqueous medium is an increasingly relevant topic. Supramolecular structures for a wide range of applications can be obtained from simple molecules, which can be easily modified to modulate the properties of the aggregates in order to yield new materials with designed properties. Surfactants that contain a metal atom in the molecular structure are named metallosurfactants (MTSs) and they are attractive molecules due to the fact that they can lead to characteristic supramolecular aggregates (micelles, vesicles, etc.), and simultaneously they contain metallic atoms. The presence of the metal makes it possible to use these aggregates in a broad range of applications such as catalysis, optoelectronics, and biomedicine.1 Although in most of the reported MTS the metallic atom is located in the polar group of the molecule, we have recently reported organometallic MTSs wherein the metallic fragment is embedded in the hydrophobic part of the molecule.2 These MTSs render mostly unilamellar vesicles in water if the MTS concentration is higher than the critical value. However, dilution of these suspensions leads to disaggregation of the vesicles, which is a drawback of using them in in vivo applications. To circumvent this disadvantage we considered the possibility of obtaining mixed systems with phospholipids. In particular, we used soybean phosphatidylcholine (SPC), a natural non-toxic phospholipid that is known to form the so-called liposomes, that is, dilution-stable closed vesicles with entrapped water (Scheme 1), and which are used for several medical applications.3 Our hypothesis is that mixed systems of MTS and phospholipids (Scheme 1) could lead to new supramolecular aggregates with a higher biocompatibility and with useful properties for biomedical applications. Since these new systems contain metallic atoms in the liposome membrane, they can be classified as a new kind of metallosome.4 In this article, we report the study of the viability of the preparation of metallosomes by mixing SPC with two organometallic MTSs, the molybdenum pentacarbonyl Mo(CO)5L (PCO) or the molybdenum tetracarbonyl complex Mo(CO)4L2 (TCO) (L = Ph2PCH2CH2SO3Na). It is known that the liposome membrane allows the inclusion of a wide variety of lipophilic or amphipathic substances such as, drugs and proteins. Thus, the incorporation of a bilayer-forming metallosurfactant into a phospholipid bilayer was considered to be a rational choice. The simplest procedure to obtain mixed vesicles involves the preparation of a homogeneous dry thin film of the substances by rotary evaporation of an organic solution, subsequent addition of aqueous medium and finally vortexing. Under these conditions the system spontaneously generates the most stable aggregates formed by intimate mixing of these substances. In our case, and as determined by dynamic light scattering (DLS) analysis, a progressive increase of the MTS/SPC molar ratio produced a significant change in the size distribution of the aggregates (Fig. 1A and B). As expected, pure SPC ([MTS] = 0) forms liposomes with a diameter of 1000 nm or greater but, when they are prepared with MTS, the size of the aggregates decreases with increasing MTS concentration. Thus, the liposome bilayer is affected by the presence of the MTS, which is indicative of the interaction of the MTS with the liposome membrane. It should be noted that there is a break point at about 3 and 10 mM for TCO (Fig. 1A) and PCO (Fig. 1B), respectively. Beyond these concentrations, very small aggregates are formed which, based on their size (about 10 nm), are compatible with the formation of micellar or bicellar structures. In fact, the addition of Triton X-100 to the suspensions at solubilising concentrations causes the disruption of the vesicles and the formation of mixed micelles of about one order of magnitude smaller (Fig. S1, ESI†). In contrast, when the surfactant is added to the small aggregates, there is only a decrease of a few nanometers in size (Fig. S1, ESI†). In addition, all the former aggregates showed physical stability at room temperature and 4 °C for at least 15 days, as determined by DLS (Fig. S1, ESI†).
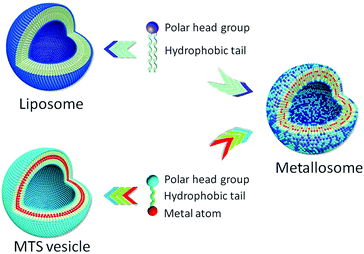 | ||
| Scheme 1 Representation of vesicles obtained with a pure phospholipid or with a metallosurfactant, and when they are mixed. | ||
The nature of the aggregates was also studied by other independent techniques. Cryo-TEM images reveal the presence of mostly unilamellar vesicles in the first range of the MTS/SPC ratio, and the presence of very small structures in the second (Fig. 1C). The existence of unilamellar vesicles can be explained by the incorporation of a negative substance into the liposome bilayer. Z-potential measurements (Table S1, ESI†) show that the incorporation of MTS into the liposome increases the negative charge of the resulting aggregates. This negative charge causes intermembrane repulsions and, consequently, promotes the formation of unilamellar aggregates. The results obtained with the previous techniques are congruent and indicate that, first, despite the differences of size and shape, MTS and SPC co-aggregate in a bilayer arrangement and, second, that at a given critical ratio, these differences collapse the membrane and beyond this point only mixed small structures are formed.
The stability of both mixed vesicles and small aggregates upon dilution was checked by DLS measurements. The progressive dilution (two orders of magnitude) of concentrated samples showed no change in the size of the aggregates, either vesicles or small aggregates, formed with TCO or PCO (Fig. 1D). Moreover, this stability was maintained for, at least, 24 hours (Fig. S2, ESI†). The same stability upon dilution was observed when the MTS/SPC vesicles were previously extruded using membranes with a pore size of 100 nm (Fig. S2C and D, ESI†). Different controls were employed. The negative ones were suspensions of SPC liposomes (extruded through 100 or 1000 nm pore-membranes), which, as expected, showed no vesicle disaggregation. The positive control was a bicellar suspension (an initial diameter of about 20 nm), which is known to disaggregate upon dilution and form micron-sized liposomes (Fig. S3, ESI†).5
At this point, our results show that MTS/SPC systems allow stable aggregates that contain metal atoms in their hydrophobic domain to be obtained. The MTS to SPC molar ratio modulates the type and size of these aggregates, since vesicles (metallosomes) and small structures of about 10 nm can be obtained. Thus, it is possible to obtain large metallosomes of hundreds of nanometers with an aqueous compartment that, in turn, can also entrap water soluble substances. In order to obtain a homogeneous vesicle distribution with controlled size, these metallosomes can be downsized by several standard methods, as is the case of liposomes (extrusion, homogenization, etc.).6 Moreover, the presence of phospholipids opens the way for the preparation of surface modified aggregates by derivatization.7 At the same time, the sulphonate group of MTS enables these metal-containing structures to incorporate, for example, polymers with amino groups, as is the case of chitosan, since a strong interaction between both the groups has been described.8 Thus, a broad spectrum of possibilities is on the horizon of these new metal structures.
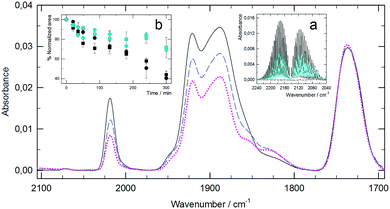
One of these opportunities is the use of MTS as carbon monoxide releasing molecules (CORMs). It has been shown that the local delivery of CO has important uses in medicine.9,10 In our case, the metal atom (Mo) of the MTS is linked to several CO groups and these molecules are good candidates to release CO under certain conditions. Therefore, a study was carried out on the PCO and TCO metal complexes, as well as their respective metallosomes and small aggregates, as CORMs. Since there is controversy about some classical assays for the detection of CO release,10,11 in order to circumvent these problems we have detected the release of CO from PCO and TCO aggregates with SPC into a CaF2 infrared cell. First, as a reference, the cell was filled with CO, and the well-known vibration–rotation spectrum of CO was obtained (inset a, Fig. 2). After eliminating the CO, the required volume of the samples was placed into the cell so that the infrared beam passed through the unfilled part when maintained in a vertical position. When the sample was kept in the dark, the acquired spectra showed no presence of CO (inset a, Fig. 2). However, after illuminating the samples with visible or UVA light, the presence of CO in the gas phase was clearly detected. Thus, our MTS can be classified as photo-CORMs, and the release of CO can be modulated via light irradiation. Once the release of CO was confirmed, its evolution was monitored by FTIR spectroscopy using dry aliquots of samples taken during irradiation. Fig. 2 shows the spectra corresponding to TCO/SPC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mol/mol before and during irradiation with UVA. As can be observed, there is a noticeable change in the CO bands centered at 2019, 1921 and 1888 cm−1, as a result of the release of CO. Similar spectra with other characteristic CO bands were obtained in the case of PCO mixed systems (Fig. S4, ESI†). These changes in the CO bands were characterized using the normalized area ratio between the MTS CO bands (2071 cm−1 and 2019 cm−1 for PCO and TCO, respectively) and the SPC CO band at 1730 cm−1, since this group is not altered during irradiation. Inset b in Fig. 2 (PCO mixed systems) indicates that the type of molecular assembly has no influence on CO release. It is also noticeable that not only UVA irradiation triggers CO release, but the gas is also produced upon illumination with visible light.
3 mol/mol before and during irradiation with UVA. As can be observed, there is a noticeable change in the CO bands centered at 2019, 1921 and 1888 cm−1, as a result of the release of CO. Similar spectra with other characteristic CO bands were obtained in the case of PCO mixed systems (Fig. S4, ESI†). These changes in the CO bands were characterized using the normalized area ratio between the MTS CO bands (2071 cm−1 and 2019 cm−1 for PCO and TCO, respectively) and the SPC CO band at 1730 cm−1, since this group is not altered during irradiation. Inset b in Fig. 2 (PCO mixed systems) indicates that the type of molecular assembly has no influence on CO release. It is also noticeable that not only UVA irradiation triggers CO release, but the gas is also produced upon illumination with visible light.
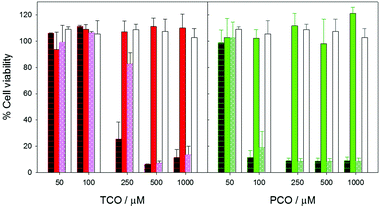
Finally, two types of tests were carried out to characterize the interaction of the MTS/SPC aggregates with biological systems. First, their 24 h-toxicity was checked by the XTT assay using human fibroblast cell cultures. The results are shown in Fig. 3, providing evidence that all the vesicular structures (metallosomes) are much less toxic than the corresponding small aggregates or the free forms of the MTS, and that PCO is more toxic than TCO when it is added as free MTS or as small aggregates.
Notably, metallosomes with TCO and PCO show no toxicity in the studied range, with their behaviour being equivalent to that of pure liposomes. The maximum MTS concentration of these systems is 1000 μM, that is, 10 and 20 times the maximum non-toxic concentrations, respectively, of TCO and PCO systems constituted of small aggregates.
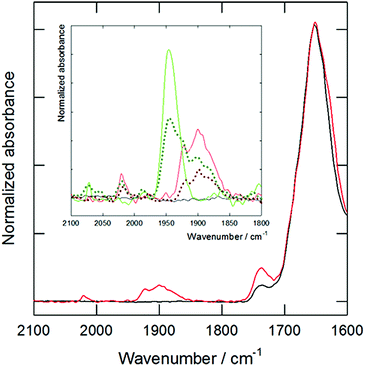
The second study was carried out to check the ability of the mixed MTS/SPC systems to act as an efficient drug delivery system. The objective was to detect the presence of MTS in cells treated with formulations containing TCO at 250 and 1000 μM, and PCO at 100 and 1000 μM. The cells were analyzed by micro-FTIR microscopy using synchrotron radiation (ALBA synchrotron, MIRAS beamline, Spain). The characteristic CO vibration band of the MTS was detected in the cells incubated at 1 mM of TCO or PCO (both as metallosomes or small aggregates), and a low intensity band was also observed in some cells treated with TCO metallosomes at 250 μM. Fig. 4 shows the results for the TCO/SPC metallosomes normalized to the total protein content of the samples (amide I band at 1650 cm−1), providing evidence of the presence of the MTS in the cells. Thus, it is clear that metallosomes are able to interact and to deliver their content to the cells.
In conclusion, we have demonstrated that the simple mixing of MTS and phospholipids leads to the formation of stable metallosomes. The reported new nanosystems are stable upon dilution and, although the incorporation of metal into cells has been observed by synchrotron radiation, a drastic decrease in cell toxicity is also shown in comparison with free MTS. Due to its simplicity and versatility, this new approach could be applied to the preparation of other metal based pharmaceuticals with low toxicity.
This work was supported by Spanish MINECO-FEDER grants (BIO2015-67358-C2-2-P and CTQ2015-70371-REDT) and by Generalitat de Catalunya (2014SGR-423). This work is dedicated to the memory of Prof. Silvia Atrian, who was the leader of our group and prematurely passed away in the winter of 2016.
Notes and references
- (a) P. C. Griffiths, I. A. Fallis, T. Chuenpratoom and R. Watanesk, Adv. Colloid Interface Sci., 2006, 122, 107–117 CrossRef CAS PubMed; (b) T. Owen and A. Butler, Coord. Chem. Rev., 2011, 255, 678–687 CrossRef CAS PubMed; (c) R. Kaur and S. K. Mehta, Coord. Chem. Rev., 2014, 262, 37–54 CrossRef CAS; (d) A. Mechler, B. D. Stringer, M. S. H. Mubin, E. H. Doeven, N. W. Phillips, J. Rudd-Schmidt and C. F. Hogan, Biochim. Biophys. Acta, 2014, 1838, 2939–2946 CrossRef CAS PubMed; (e) L. D. Wickramasinghe, S. Mazumder, S. Gonawala, M. M. Perera, H. Baydoun, B. Thapa, L. Li, L. Xie, G. Mao, Z. Zhou, H. B. Schlegel and C. N. Verani, Angew. Chem., Int. Ed., 2014, 53(52), 14462–14467 CrossRef CAS PubMed; (f) Y. Chen, Q. Zhu, X. Cui, W. Tang, H. Yang, Y. Yuan and A. Hu, Chem. – Eur. J., 2014, 20(39), 12477–12482 CrossRef CAS PubMed; (g) L. J. Lalgee, L. Griersona, R. A. Fairman, G. E. Jaggernauth, A. Schulte, R. Benz and M. Winterhalter, Biochim. Biophys. Acta, 2014, 1838, 1247–1254 CrossRef CAS PubMed.
- (a) E. Parera, F. Comelles, R. Barnadas and J. Suades, Chem. Commun., 2011, 47, 4460–4462 RSC; (b) E. Parera, M. Marín-García, R. Pons, F. Comelles, J. Suades and R. Barnadas-Rodríguez, Organometallics, 2016, 35, 484–493 CrossRef CAS.
- (a) Y. Perrie, F. Crofts, A. Devitt, H. R. Griffiths, E. Kastner and V. Nadella, Adv. Drug Delivery Rev., 2016, 99A, 85–96 CrossRef PubMed; (b) B. S. Pattni, V. V. Chupin and V. P. Torchilin, Chem. Rev., 2015, 115, 10938–10966 CrossRef CAS PubMed.
- (a) J. F. Hainfeld, F. R. Furuya and R. D. Powell, J. Struct. Biol., 1999, 127(2), 152–160 CrossRef CAS PubMed; (b) K. Osada, H. Cabral, Y. Mochida, S. Lee, K. Nagata, T. Matsuura, M. Yamamoto, Y. Anraku, A. Kishimura, N. Nishiyama and K. Kataoka, J. Am. Chem. Soc., 2012, 134, 13172–13175 CrossRef CAS PubMed.
- M. Li, H. H. Morales, J. Katsaras, N. Kucerka, Y. Yang, P. M. Macdonald and M.-P. Nieh, Langmuir, 2013, 29, 15943–15957 CrossRef CAS PubMed.
- (a) A. Hinna, F. Steiniger, S. Hupfeld, P. Stein, J. Kuntsche and M. Brandl, J. Liposome Res., 2015, 26(1), 11–20 CrossRef PubMed; (b) R. Barnadas and M. Sabés, Methods Enzymol., 2003, 367, 28–46 Search PubMed.
- (a) A. S. Manjappa, K. R. Chaudhari, M. P. Venkataraju, P. Dantuluri, B. Nanda, Ch. Sidda, K. K. Sawant and R. S. Murthy, J. Controlled Release, 2011, 150, 2–22 CrossRef CAS PubMed; (b) A. Accardo and G. Morelli, Biopolymers, 2015, 104(5), 462–479 CrossRef CAS PubMed; (c) R. R. Sawant and V. P. Torchilin, AAPS J., 2012, 14(2), 303–315 CrossRef CAS PubMed.
- (a) Q. Yua, S. Denga and G. Yu, Water Res., 2008, 42, 3089–3097 CrossRef PubMed; (b) R. Barnadas-Rodríguez, Macromol. Chem. Phys., 2013, 213, 99–106 CrossRef.
- (a) R. Motterlini and L. E. Otterbein, Nat. Rev. Drug Discovery, 2010, 9(9), 728–743 CrossRef CAS PubMed; (b) A. C. Kautz, P. C. Kunz and C. Janiak, Dalton Trans., 2016, 45, 18045–18063 RSC; (c) U. Hasegawa, A. J. van der Vlies, E. Simeoni, Ch. Wandrey and J. A. Hubbell, J. Am. Chem. Soc., 2010, 132, 18273–18280 CrossRef CAS PubMed.
- G. J. L. Bernardes and S. García-Gallego, Angew. Chem., Int. Ed., 2014, 53, 9712–9721 CrossRef PubMed.
- (a) S. McLean, B. E. Mann and R. K. Poole, Anal. Biochem., 2012, 427, 36–40 CrossRef CAS PubMed; (b) S. H. Heinemann, T. Hoshi, M. Westerhausend and A. Schiller, Chem. Commun., 2014, 50, 3644–3660 RSC; (c) M. Chaves-Ferreira, I. S. Albuquerque, D. Matak-Vinkovic, A. C. Coelho, S. M. Carvalho, L. M. Saraiva, C. C. Romão and G. J. L. Bernardes, Angew. Chem., Int. Ed., 2015, 54, 1172–1175 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods with experimental details, supporting Fig. S1–S4. See DOI: 10.1039/c7cc04945e |
| This journal is © The Royal Society of Chemistry 2017 |