Self-assembly of colloidal molecules that respond to light and a magnetic field†
Abstract
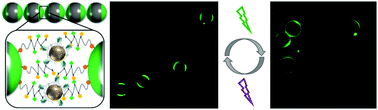
This communication reports a novel method to prepare Janus particles with light-responsive arylazopyrazole (AAP) polymer caps, which can be reversibly cross-linked to chain-like colloidal oligomers in the presence of cyclodextrin (CD) functionalized superparamagnetic nanoparticles. The resulting colloidal molecules are light-responsive and can be controlled by an external magnetic field.


 Please wait while we load your content...
Please wait while we load your content...