Highly efficient Cr2O72− removal of a 3D metal-organic framework fabricated by tandem single-crystal to single-crystal transformations from a 1D coordination array†
Abstract
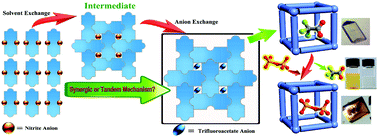
The single-crystal to single-crystal (SC–SC) transformation from a 1D coordination chain to a 3D coordination network, which is triggered by both solvent and anion exchanges, has been revealed to suffer from a tandem mechanism as proved by isolation of the intermediate state. The resulting porous crystalline material shows a high efficiency for the capture of dichromates (207 mg g−1) via the SC–SC anion-exchange.


 Please wait while we load your content...
Please wait while we load your content...