Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Scavenger templates: a systems chemistry approach to the synthesis of porphyrin-based molecular wires†
Arjen
Cnossen
,
Cécile
Roche
and
Harry L.
Anderson
 *
*
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Oxford, OX1 3TA, UK. E-mail: harry.anderson@chem.ox.ac.uk
First published on 7th September 2017
Abstract
A hexa-pyridyl template can be used as a scavenger to facilitate the synthesis of a linear porphyrin dodecamer from a mixture of linear hexamers with one or two terminal reactive groups. The template suppresses polymer formation by rapidly cyclizing the fully deprotected hexamer, thus up-regulating formation of the linear dodecamer.
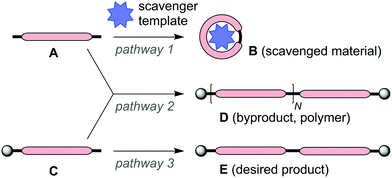
Complex networks of chemical reactions determine the behaviour of living cells.1 Even simple networks of a few reactions can display surprising emergent behaviour such as bistability and temporal oscillations, and the study of these effects is known as ‘systems chemistry’.2–6 A key principle of systems chemistry is that in a network of reactions up-regulation of one reaction pathway can change the flux of material through another reaction pathway, even when, at first sight, the two reactions are unconnected. This idea is easily appreciated by considering the example of three irreversible reactions shown in Scheme 1. Increasing the rate constant k1 (while keeping k2 and k3 constant) increases the fraction of A that is converted to B because rapid conversion of A to B reduces the concentration of A, suppressing formation of D. Increasing k1 also increases the fraction of C that is converted to E, since the competing formation of D is suppressed. Up-regulating pathway 1 has the effect of up-regulating pathway 3; these two reactions are coupled because both A and C participate in pathway 2.
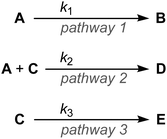 | ||
| Scheme 1 In this general network of three reactions, up-regulation of pathway 1 causes up-regulation of pathway 3. | ||
The approach to indirect up-regulation summarized in Scheme 1 was applied to template-directed synthesis by Sanders and co-workers in 1992, in a strategy called ‘scavenger templating’ (Scheme 2).7 In this system, the desired reaction is dimerization of C to give E (pathway 3), but the monofunctional compound C is difficult to separate from a bifunctional analogue A, and in the absence of the template, A couples with C to form D (pathway 2), reducing the yield of E. In the presence of the template, A undergoes rapid cyclization to give B (pathway 1), indirectly up-regulating the conversion of C into E, and increasing the yield of the desired product. Here we demonstrate the application of this approach to favor the formation of a butadiyne-linked π-conjugated porphyrin dodecamer P12Si2 (structure shown in Fig. 1).
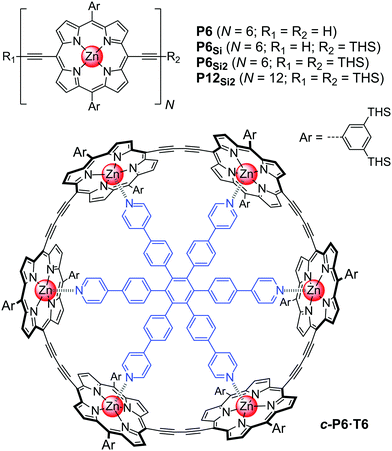
Conjugated porphyrin oligomers such as P12Si2 have a variety of unusual optical properties,8,9 and they are described as ‘molecular wires’ because of their ability to mediate long-range charge transport.10–12 These mono-disperse oligomers are generally synthesized via an iterative process, using partial deprotection of bis-acetylenic building blocks, chromatographic isolation of the mono-deprotected compound and oxidative Glaser coupling.9,13 The chromatographic separation of the mixture of protected and deprotected alkynes is the limiting step, and in principle this problem can be avoided by using a scavenger template. Previous work has shown that the hexapyridyl template T6 effectively directs the cyclization of the linear porphyrin hexamer P6 to form the cyclic hexamer template complex c-P6·T6 (Fig. 1).14 Here we show that T6 can be used as a scavenger template in the synthesis of P12Si2 and we analyze the factors determining the efficiency of this strategy.
This scavenger templating system is summarized in Scheme 3. When an inseparable mixture of the fully-deprotected, mono-protected, and fully-protected porphyrin hexamers (P6, P6Si and P6Si2) is subjected to Glaser coupling, the product is a polydisperse mixture of linear oligomers, together with unreacted P6Si2 (Scheme 3, left). Addition of T6 to the initial mixture of P6, P6Si and P6Si2 leads to the formation of complexes in which the linear hexamers are forced into cyclic conformations. Under Glaser coupling conditions, the fully deprotected hexamer P6 is rapidly cyclized to form c-P6·T6. This leaves mono-deprotected P6Si to dimerize, yielding the desired dodecamer P12Si2 (Scheme 3, right).
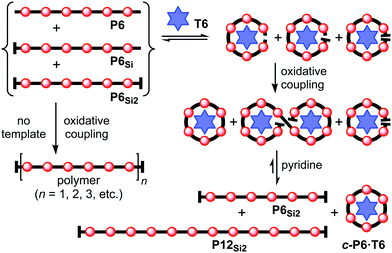 | ||
| Scheme 3 Use of T6 as a scavenger template in the synthesis of the porphyrin dodecamer P12Si2. Coupling conditions: benzoquinone, Pd(PPh3)2Cl2, CuI, i-Pr2NH, CHCl3, 20 °C. | ||
Partial deprotection of hexamer P6Si2 was achieved by treatment with tetra-n-butylammonium fluoride (TBAF) while monitoring the reaction by 1H NMR spectroscopy to give a mixture of P6Si2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P6Si
P6Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P6 in the ratio of approximately 1
P6 in the ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
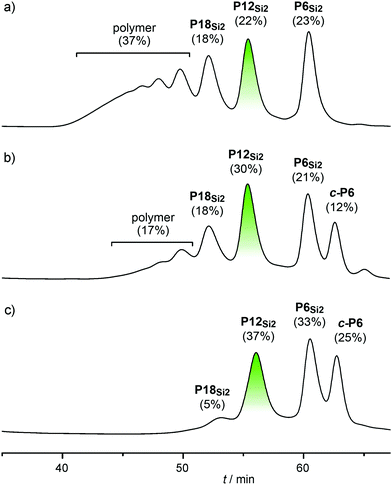
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. When the mixture was subjected to palladium-catalyzed Glaser coupling in the absence of template, the expected statistical mixture of oligomers was obtained, as determined by analytical gel permeation chromatography (GPC, Fig. 2a). We use palladium catalysis in these Glaser coupling reactions because these reaction conditions give essentially quantitative coupling at room temperature, even at low concentration.15 In the presence of the template, the yield of P12Si2 increases from 22% to 30%, with concomitant formation of cyclic hexamer c-P6·T6 and a strong decrease in the amount of polymeric by-products (Fig. 2b). We used 2 equivalents of T6, relative to the total amount of hexamer (P6Si2 + P6Si + P6), so that all the hexamer is bound to the template. During this reaction, there is a competition between intramolecular cyclization of P6·T6, to form c-P6·T6, and bimolecular coupling between P6·T6 and P6Si·T6, which leads to the formation of polymer. Thus one would expect the effect of the scavenger template to be greater at lower concentrations, and this was confirmed experimentally: running the reaction at a lower concentration (0.2 mM rather than 2 mM) increases the yield of P12Si2 to 37%, while increasing the yield of c-P6·T6 to 25% and totally suppressing polymer formation (Fig. 2c). Dilution has no detectable effect on the product distribution in the absence of T6, however the coupling reaction becomes slow under dilute conditions, necessitating the addition of several portions of catalyst over time.
1. When the mixture was subjected to palladium-catalyzed Glaser coupling in the absence of template, the expected statistical mixture of oligomers was obtained, as determined by analytical gel permeation chromatography (GPC, Fig. 2a). We use palladium catalysis in these Glaser coupling reactions because these reaction conditions give essentially quantitative coupling at room temperature, even at low concentration.15 In the presence of the template, the yield of P12Si2 increases from 22% to 30%, with concomitant formation of cyclic hexamer c-P6·T6 and a strong decrease in the amount of polymeric by-products (Fig. 2b). We used 2 equivalents of T6, relative to the total amount of hexamer (P6Si2 + P6Si + P6), so that all the hexamer is bound to the template. During this reaction, there is a competition between intramolecular cyclization of P6·T6, to form c-P6·T6, and bimolecular coupling between P6·T6 and P6Si·T6, which leads to the formation of polymer. Thus one would expect the effect of the scavenger template to be greater at lower concentrations, and this was confirmed experimentally: running the reaction at a lower concentration (0.2 mM rather than 2 mM) increases the yield of P12Si2 to 37%, while increasing the yield of c-P6·T6 to 25% and totally suppressing polymer formation (Fig. 2c). Dilution has no detectable effect on the product distribution in the absence of T6, however the coupling reaction becomes slow under dilute conditions, necessitating the addition of several portions of catalyst over time.
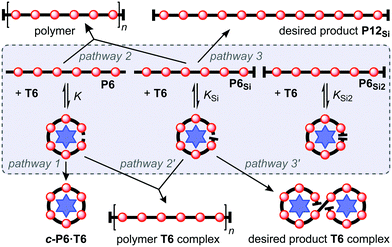
The network of reversible and irreversible reactions involved in this system is shown in Scheme 4. The main role of the T6 template is to open a new reaction channel (pathway 1) to scavenge P6 from the system. The template may also reduce the rates of pathways 2′ and 3′ (compared with 2 and 3), by steric hindrance of coupling of template-bound oligomers. This steric effect may be beneficial, by making pathway 1 compete more effectively with pathway 2′, but it could also become a problem, if it makes pathway 3′ so slow that formation of the desired product does not reach completion. We were interested to test the relative values of the binding constants K, KSi and KSi2, because the bulky terminal THS groups might be expected to hinder template binding in P6Si and P6Si2. These binding constants are too high to measure by direct titration, but they can be determined by denaturing the complexes with pyridine.16 UV-vis-NIR titrations of P6·T6 and P6Si2·T6 with pyridine gave log(K) = 18.49 ± 0.21 and log(KSi2) = 18.08 ± 0.27 (see ESI† for details). Thus K appears to be slightly larger than KSi2, as expected, but the difference is comparable to the experimental uncertainty. We also measured the relative affinities of P6 and P6Si2 for T6 by carrying out a 1H NMR competition experiment in which T6 was titrated into a solution of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of P6 and P6Si2 in CDCl3. The mixture of P6, P6Si2, P6·T6 and P6Si2·T6 is in slow exchange on the NMR timescale and the signals from the terminal β-pyrrole protons of the four species can be resolved; integration of these signals indicates that the ratio of the binding constants is K/KSi2 = 1.95 ± 0.13 (see ESI† for details). This experiment confirmed that the deprotected hexamer binds the template more strongly than the protected hexamer, although the effect is surprisingly subtle.
1 mixture of P6 and P6Si2 in CDCl3. The mixture of P6, P6Si2, P6·T6 and P6Si2·T6 is in slow exchange on the NMR timescale and the signals from the terminal β-pyrrole protons of the four species can be resolved; integration of these signals indicates that the ratio of the binding constants is K/KSi2 = 1.95 ± 0.13 (see ESI† for details). This experiment confirmed that the deprotected hexamer binds the template more strongly than the protected hexamer, although the effect is surprisingly subtle.
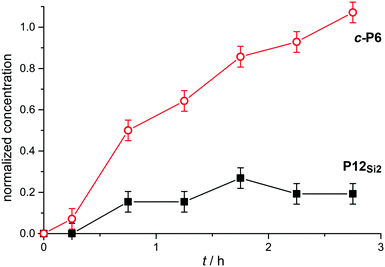
We attempted to carry out kinetic experiments to test whether pathway 1 is actually faster than pathways 2′ and 3′ (Scheme 4). It is difficult to follow the kinetics of these reactions because fresh aliquots of catalyst need to be added over time to take the reaction to completion, however we were able to follow the initial stage of the reaction using analytical GPC. The results of this experiment (Fig. 3) confirm that formation of c-P6·T6 is faster than formation of P12Si2, as expected for scavenger templating.
In summary, we have demonstrated that a hexapyridyl ligand T6 acts as a scavenger template to promote the synthesis of a monodisperse π-conjugated linear porphyrin dodecamer P12Si2. The template prevents polymerization, thereby increasing the yield of the desired product from 22% to 37% (analytical GPC yields). The conventional alternative to using a scavenger template is to separate the mixture of linear hexamers (P6, P6Si and P6Si2) or to carry out the coupling on the mixture of hexamers and to separate all the linear oligomeric products.17 Recent advances in recycling HPLC and the development of polar protecting groups for alkynes17–19 facilitate these separation strategies, but the use of a scavenger template is more biomimetic: nature does not create a required molecular structure by synthesizing complex mixtures of products then using the equivalent of HPLC to separate and discard unwanted species. This work illustrates the scope for creating artificial systems that control the outcome of complex networks of reactions by indirectly up-regulating specific pathways.
This project was supported by the EPSRC and the ERC (grant 320969).
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Albert, J. Cell Sci., 2005, 118, 4947–4957 CrossRef CAS PubMed.
- J. R. Nitschke, Nature, 2009, 462, 736–738 CrossRef CAS PubMed.
- R. F. Ludlow and S. Otto, Chem. Soc. Rev., 2008, 37, 101–108 RSC.
- G. Ashkenasy, T. M. Hermans, S. Otto and A. F. Taylor, Chem. Soc. Rev., 2017, 46, 2543–2554 RSC.
- S. N. Semenov, L. J. Kraft, A. Ainla, M. Zhao, M. Baghbanzadeh, V. E. Campbell, K. Kang, J. M. Fox and G. M. Whitesides, Nature, 2016, 537, 656–660 CrossRef CAS PubMed.
- V. del Amo and D. Philp, Chem. – Eur. J., 2010, 16, 13304–13318 CrossRef CAS PubMed.
- S. Anderson, H. L. Anderson and J. K. M. Sanders, Angew. Chem., Int. Ed., 1992, 31, 907–910 CrossRef.
- M. Drobizhev, Y. Stepanenko, A. Rebane, C. J. Wilson, T. E. O. Screen and H. L. Anderson, J. Am. Chem. Soc., 2006, 128, 12432–12433 CrossRef CAS PubMed.
- P. Parkinson, D. V. Kondratuk, C. Menelaou, J. Q. Gong, H. L. Anderson and L. M. Herz, J. Phys. Chem. Lett., 2014, 5, 4356–4361 CrossRef CAS PubMed.
- M. U. Winters, E. Dahlstedt, H. E. Blades, C. J. Wilson, M. J. Frampton, H. L. Anderson and B. Albinsson, J. Am. Chem. Soc., 2007, 129, 4291–4297 CrossRef CAS PubMed.
- G. Sedghi, V. M. García-Suárez, L. J. Esdaile, H. L. Anderson, C. J. Lambert, S. Martin, D. Bethell, S. J. Higgins, M. Elliott, N. Bennett, J. E. Macdonald and R. J. Nichols, Nat. Nanotechnol., 2011, 6, 517–523 CrossRef CAS PubMed.
- R. C. Bruce, R. Wang, J. Rawson, M. J. Therien and W. You, J. Am. Chem. Soc., 2016, 138, 2078–2081 CrossRef CAS PubMed.
- P. N. Taylor, J. Huuskonen, G. Rumbles, R. T. Aplin, E. Williams and H. L. Anderson, Chem. Commun., 1998, 909–910 RSC.
- M. Hoffmann, J. Kärnbratt, M. H. Chang, L. M. Herz, B. Albinsson and H. L. Anderson, Angew. Chem., Int. Ed., 2008, 47, 4993–4996 CrossRef CAS PubMed.
- V. E. Williams and T. M. Swager, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4669–4676 CrossRef CAS.
- H. J. Hogben, J. K. Sprafke, M. Hoffmann, M. Pawlicki and H. L. Anderson, J. Am. Chem. Soc., 2011, 133, 20962–20969 CrossRef CAS PubMed.
- N. Kamonsutthipaijit and H. L. Anderson, Chem. Sci., 2017, 8, 2729–2740 RSC.
- S. Höger and K. Bonrad, J. Org. Chem., 2000, 65, 2243–2245 CrossRef.
- G. Gaefke and S. Höger, Synthesis, 2008, 2155–2157 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, GPC traces and spectroscopic data. See DOI: 10.1039/c7cc04289b |
| This journal is © The Royal Society of Chemistry 2017 |