Chemically individual armoured bioreporter bacteria used for the in vivo sensing of ultra-trace toxic metal ions†
Abstract
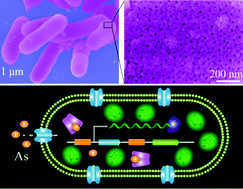
A chemically engineered armour is developed for simultaneously improving bioreporter bacterial vitality and shielding infectivity. The armour can help bacteria to resist various insults and even immune phagocytosis. Meanwhile, the bacterial infectivity has proven to be greatly shielded as well. Most importantly, the original bacterial biosensing activity is well preserved, which is competent for sensing trace arsenic in water, serum, and even in vivo.


 Please wait while we load your content...
Please wait while we load your content...