Extremely low catalyst loading for electroless deposition on a non-conductive surface by a treatment for reduced graphene oxide†
Abstract
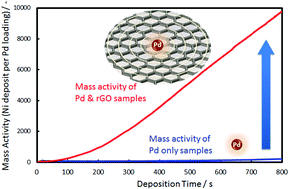
Enhancement of electroless deposition using reduced-graphene-oxide (rGO) coating for a conductive surface on the non-conductive substrate is proposed and demonstrated. We confirmed that the rGO treatment enhanced the Pd catalytic activity and achieved a sufficient rate in electroless deposition with extremely low Pd loading (3.94 × 10−2 μg cm−2).


 Please wait while we load your content...
Please wait while we load your content...