Open Access Article
Open Access ArticleDesorption dynamics of CO2 from formate decomposition on Cu(111)†
Fahdzi
Muttaqien
 *a,
Hiroyuki
Oshima
a,
Yuji
Hamamoto
ab,
Kouji
Inagaki
ab,
Ikutaro
Hamada
*a,
Hiroyuki
Oshima
a,
Yuji
Hamamoto
ab,
Kouji
Inagaki
ab,
Ikutaro
Hamada
 b and
Yoshitada
Morikawa
b and
Yoshitada
Morikawa
 *abc
*abc
aDepartment of Precision Science and Technology, Graduate School of Engineering, Osaka University, 2-1, Yamada-oka, Suita, Osaka 565-0871, Japan. E-mail: fahdzi@cp.prec.eng.osaka-u.ac.jp; morikawa@prec.eng.osaka-u.ac.jp
bElements Strategy Initiative for Catalysts and Batteries (ESICB), Kyoto University, Katsura, Kyoto 615-8520, Japan
cResearch Center for Ultra-Precision Science and Technology, Graduate School of Engineering, Osaka University, Japan
First published on 2nd August 2017
Abstract
We performed ab initio molecular dynamics analysis of formate decomposition to CO2 and H on a Cu(111) surface using van der Waals density functionals. Our analysis shows that the desorbed CO2 has approximately twice larger bending vibrational energy than the translational, rotational, and stretching vibrational energies. Since formate synthesis, the reverse reaction of formate decomposition, has been suggested experimentally to occur via the Eley–Rideal mechanism, our results indicate that the formate synthesis can be enhanced if the bending vibrational mode of CO2 is excited rather than the translational and/or stretching vibrational modes. Detailed information on the energy distribution of desorbed CO2 as a formate decomposition product may provide new insights for improving the catalytic activity of formate synthesis.
One of the most important intermediate steps in the methanol synthesis over Cu-based catalysts is CO2 hydrogenation into formate (HCOO).1–5 Based on a kinetics analysis, it was clarified that formate synthesis is structure insensitive and consistent with an Eley–Rideal (ER) type mechanism.6–9 Recently, theoretical investigations also suggested the ER and hot atom (HA) mechanisms during CO2 reduction into formate and/or hydrocarboxyl (HOCO).10 The ER type mechanism suggests that the formate synthesis can be enhanced by controlling the translational, vibrational, and rotational energies of impinging CO2. The initial impinging CO2 energies must be related to the energy states of desorbed CO2 from formate decomposition because formate synthesis and decomposition are reverse reactions. Therefore, the elucidation of formate decomposition dynamics is important for improving catalytic formate synthesis.
Very recently, the dynamics of desorbed CO2 produced from the formate decomposition reaction has been studied by measuring the angle-resolved intensity and translational energy distribution of the steady-state reaction of formic acid (HCOOH) and oxygen on a Cu surface.11 It was reported that the translational energy of desorbed CO2 is approximately 0.10 eV and independent of the surface temperature. The measured CO2 translational energy is much smaller than the activation energy of formate synthesis, which is 0.59 ± 0.05 eV.7–9 This suggests that internal modes, such as vibrational and rotational modes, should be excited in the desorbed CO2. In this study, we therefore investigate the energy distribution of desorbed CO2 as a formate decomposition product by means of ab initio calculations. We also show the importance of the van der Waals (vdW) interaction in determining the energetics of desorbed CO2. From the knowledge of desorbing product dynamics, we can deduce optimal conditions for the formate synthesis reaction through CO2 hydrogenation.
The calculations were performed using a plane-wave pseudopotential code named “Simulation tool for Atom TEchnology (STATE)”.12 Since the vdW interaction is important to describe the adsorption and chemical reaction of inert molecules,13 we compare the Perdew–Burke–Ernzerhof (PBE) results with those obtained using vdW density functionals (vdW-DFs), i.e., the original vdW-DF (vdW-DF1),14 optB86b-vdW,15 and rev-vdW-DF216 functionals as implemented in the STATE code.17 We also included the dispersion correction proposed by Grimme with PBE (PBE-D2).18 We performed ab initio molecular dynamics (AIMD) simulations starting from the transition state (TS) structure of the formate decomposition process to gas phase CO2 and adsorbed H to evaluate the translational and internal (rotational and vibrational) energies of desorbed CO2. We have also considered additional samples of the initial geometry for AIMD simulations. We displaced the atoms at the TS towards the second, third, fourth, fifth, and sixth lowest normal mode vectors in such a way that the energies of the displaced geometries are increased from the energy of the TS by 0.02 eV. In the AIMD simulations, the initial velocities are given randomly and they are scaled to set the average kinetic energy of 300 K. Here, we do not include the zero-point energy in the sampling of initial conditions. The detailed calculation methods are provided in the ESI.†
We first calculated the minimum energy path of CO2 hydrogenation into bidentate formate (bi-HCOO) through monodentate formate (m-HCOO) on Cu(111). The bidentate formate has been observed experimentally as an abundant product during CO2 hydrogenation compared with its isomer (hydrocarboxyl (HOCO)).7–9 Moreover, we have also performed the calculations of HOCO adsorption on Cu(111) and found that this species is 0.89 eV less stable using one of the vdW-DFs (optB86b-vdW). Fig. 1 and Table 1 show the energy profile of CO2 hydrogenation into formate and its detailed descriptions, respectively. As shown in Fig. 1, the hydrogen chemisorption energies (the energy levels of CO2(g) + 1/2H2(g)) are quite similar among all functionals except vdW-DF1. On the other hand, the physisorption energies of CO2 (the energy levels of CO2(phys) + H*), the energies of the TSs, m-HCOO*, and bi-HCOO* are stabilized through the vdW attraction by approximately 0.13–0.37 eV relative to the PBE result. We then consider the activation energy of formate synthesis (Ea(syn)) as the energy difference between the TS and the CO2(g) + H* state. This definition of Ea(syn) should correspond to the experimentally observed activation energy of formate synthesis, in which formate was synthesized at a temperature of 333–353 K under atmospheric pressure.7 Under these conditions, the hydrogen coverage is close to the saturation point, while CO2 coverage is almost negligible due to a small adsorption energy. The activation energy of formate decomposition (Ea(dec)) is defined as the energy difference between the TS and bi-HCOO*. As shown in Table 1, only PBE overestimates the experimental Ea(syn), while each functional with a vdW interaction gives a reasonably good result. Meanwhile, each calculated Ea(dec) is in good agreement with the experimental results.
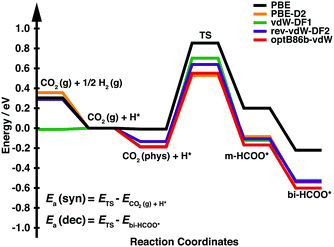 | ||
| Fig. 1 Energy profile for CO2 hydrogenation to bidentate formate on Cu(111). The energy zero is the sum of the total energies of gas phase CO2 and an adsorbed hydrogen on the surface (CO2(g) + H*). | ||
| This study | Expt. | |||||
|---|---|---|---|---|---|---|
| PBE | PBE-D2 | vdW-DF1 | rev-vdW-DF2 | optB86b-vdW | ||
| a Taken from ref. 7. b Taken from ref. 8 and 9. | ||||||
| E CO2(g) + 1/2H2(g) | +0.30 (+0.22) | +0.36 | −0.01 | +0.29 | +0.30 (+0.22) | — |
| E CO2(phys) + H* | −0.01 (−0.01) | −0.19 | −0.18 | −0.13 | −0.19 (−0.18) | — |
| E TS | +0.85 (+0.78) | +0.53 | +0.70 | +0.64 | +0.55 (+0.52) | — |
| E m-HCOO* | +0.20 (+0.17) | −0.08 | −0.12 | −0.11 | −0.17 (−0.18) | — |
| E bi-HCOO* | −0.22 (−0.33) | −0.54 | −0.52 | −0.53 | −0.60 (−0.70) | — |
| E a(syn) | 0.85 (0.78) | 0.53 | 0.70 | 0.64 | 0.55 (0.52) | |
| E a(syn) + ZPE | 0.80 (0.73) | 0.48 | 0.65 | 0.59 | 0.50 (0.47) | 0.59 ± 0.05a |
| 0.66 ± 0.02b | ||||||
| E a(dec) | 1.08 (1.12) | 1.07 | 1.23 | 1.17 | 1.15 (1.22) | |
| E a(dec) + ZPE | 0.90 (0.94) | 0.89 | 1.05 | 0.99 | 0.97 (1.04) | 1.12 ± 0.03a |
| 1.17 ± 0.13b | ||||||
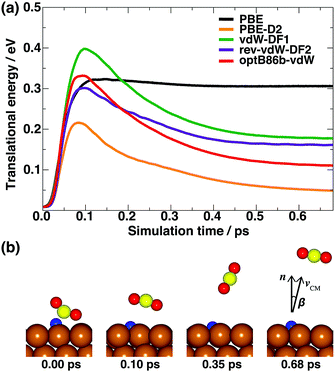
Next, we explored the energy distribution of desorbed CO2 as a product of formate decomposition. We calculated the CO2 translational energy (Et) from the velocity of the center of mass of CO2. Fig. 2a summarizes the time evolution of CO2 translational energy. The representative snapshots of CO2 desorption from the AIMD trajectory are shown in Fig. 2b. There are significant differences in the Et profiles calculated using PBE and those using PBE-D2 and vdW-DFs. Here, PBE predicts a constant Et value at 0.30 eV after the CO2 molecule experiences Pauli repulsion in the first 0.10 ps (shown as a black line in Fig. 2a). Meanwhile, the PBE-D2 and vdW-DFs show different time evolution profiles of the CO2 translational energy. In the first 0.08–0.10 ps, the Et value increases to 0.20–0.32 eV due to the Pauli repulsion between CO2 and the surface. Then, it decreases gradually to a certain value. The decrease in the CO2 translational energy is due to the vdW attraction between CO2 and the Cu(111) surface. We also estimated the desorption angle (β) of CO2 at 0.68 ps, when it already has a constant Et value, from the direction of the velocity of center of mass relative to the surface normal (Fig. 2b). The calculated β using PBE, PBE-D2, vdW-DF1, rev-vdW-DF2, and optB86b-vdW are 5°, 18°, 8°, 12°, and 14°, respectively, in which the angular distribution of desorbed CO2 shows quite sharp collimation along the surface normal direction and is in good agreement with the experimental results.11 As summarized in Table 2, the calculated Et values using PBE-D2 and vdW-DFs are in reasonable agreement with the experimental estimation,11 while PBE fails in predicting this energy. Therefore, the vdW interaction is important for describing the translational energy of desorbed CO2.
| This study | Expt.a | |||||
|---|---|---|---|---|---|---|
| PBE | PBE-D2 | vdW-DF1 | rev-vdW-DF2 | optB86b-vdW | ||
| a Experimentally measured CO2 translational energy as a formate decomposition product taken from ref. 11. b This energy is deduced from: Ea(syn) − (Et + Er + Eb + Es). | ||||||
| E t | 0.30 | 0.05 | 0.18 | 0.16 | 0.14 ± 0.03 | 0.10 |
| E r | 0.08 | 0.09 | 0.08 | 0.11 | 0.11 ± 0.03 | — |
| E b | 0.27 | 0.24 | 0.25 | 0.26 | 0.26 ± 0.02 | — |
| E s | 0.06 | 0.05 | 0.10 | 0.08 | 0.04 ± 0.02 | — |
| Surface modeb | 0.14 | 0.10 | 0.09 | 0.03 | 0.03 ± 0.02 | — |
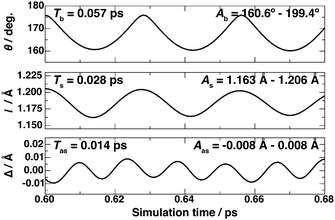
We also evaluated the energy transfer by formate decomposition into the internal modes (rotational and vibrational) of CO2. The rotational energy (Er) of CO2 as a function of time was calculated from the moment of inertia of CO2 and its angular momentum at each time step of the MD trajectory. Table 2 shows that the calculated Er value varies in between 0.08 and 0.11 eV. The CO2 bending, symmetric stretching, antisymmetric stretching vibrational energies are evaluated based on the time evolution of the desorbed CO2 geometry, i.e., bond angle (θ), the C–O bond length (l), and the difference between two C–O bond lengths (Δ), respectively, as shown in Fig. 3. For comparison, we also evaluated the vibrational frequencies of an isolated CO2 (see the ESI†). The calculated zero-point energies of isolated CO2 bending, symmetric stretching, and antisymmetric stretching vibrational modes using optB86b-vdW are 76.72 meV, 164.61 meV, and 295.74 meV, respectively, which are in good agreement with the experimental values of 82.70 meV, 165.27 meV, and 291.24 meV, respectively.19,20 These calculated zero-point energies are nearly independent of the functional. The vibrational energy of the bending mode (Eb ∼ 0.25 eV) of desorbed CO2 is close to the third excitation of the zero-point energy of isolated CO2. All functionals produce similar Eb values in the range of 0.24–0.26 eV. The calculated vibrational energy of the CO2 symmetric mode (Es) varies between 0.05 and 0.11 eV, which is close to the zero-point energy of the isolated CO2 symmetric mode. Meanwhile, the vibrational energy of the antisymmetric mode (Eas ∼ 1.5 meV) is much smaller than the zero-point energy of isolated CO2.
Based on our calculations, nearly a half of Ea(syn) is transferred into the CO2 bending mode, a quarter into each translational and rotational modes, and rather a small energy is transferred into the CO2 stretching modes. The origin for the strong enhancement of the CO2 bending mode can be ascribed to its geometry at the TS, in which the CO2 bond angle is nearly 140°. The rest of Ea(syn) may have transferred into the surface modes, namely, hydrogen–Cu and/or Cu–Cu vibrations. We also performed the sudden vector projection (SVP) analysis proposed by Jiang and Guo.21 We showed that the SVP analysis qualitatively agrees rather well with our AIMD simulation results (shown in the ESI†). Therefore, our results strongly suggest that formate synthesis, the reverse process of the formate decomposition, can be enhanced by increasing the vibrational energy of the CO2 bending mode.
The present results are in sharp contrast to the case of CO2 dissociation, in which the CO2 symmetric and antisymmetric stretching modes are theoretically suggested to be more important to increase the dissociation rate.22 The importance of stretching modes in the CO2 dissociation was implied experimentally in the study of CO2 dynamics from CO oxidation on Pd surfaces.23–25 The desorbed CO2 has 0.12–0.16 eV in the vibrational energy of the antisymmetric mode, which is slightly larger than the vibrational energy of the CO2 symmetric-bending mode (0.11–0.13 eV). Moreover, the measured translational (0.10–0.12 eV)26 and rotational (0.08–0.10 eV)23,25,27,28 energies of desorbed CO2 from CO oxidation on Pd surfaces are smaller than its vibrational energy of the antisymmetric mode. Therefore, CO2 dissociation can be selectively enhanced by exciting the CO2 stretching vibrational modes.
Finally, we discuss the temperature dependence of the translational energy of CO2. Experimentally, it was reported that Et of desorbed CO2 from formate decomposition is independent of the surface temperature,11 while that from CO oxidation is linearly dependent on the surface temperature.26 From our theoretical simulations, it turns out that at most 20% of Ea(syn) is transferred into surface modes. On the other hand, it can be deduced that approximately 60% of the activation energy of CO2 dissociation (1.30 eV)29 on a Pd surface, which is a reverse reaction of CO oxidation, is transferred to the surface mode. Both results, namely, the surface temperature dependence and the energy transfer to the surface mode, indicate the strength of the coupling between CO2 and the surfaces at the TS of reactions. In the case of formate decomposition, CO2 is weakly bonded as seen in the left panel of Fig. 2b. This is in contrast to the case of CO oxidation, where CO2 was found to spend a significant residence time in the chemisorption well before it desorbs.30 Moreover, one of the two C–O bond lengths of CO2 is significantly elongated at the TS of CO oxidation, and carbon and oxygen are strongly bonded to the surface.31
In summary, we have investigated the minimum energy path of CO2 hydrogenation into formate and the dynamics of formate decomposition into gas phase CO2 and adsorbed hydrogen using density functional theory calculations. Based on the dynamics analysis of formate decomposition, the bending energy of desorbed CO2 is twice larger than the translational energy. Since formate synthesis from CO2 and H2, the reverse reaction of the formate decomposition, is experimentally suggested to occur via the ER type mechanism, our results indicate that the reaction rate of formate synthesis can be enhanced if the bending vibrational mode of CO2 is excited rather than the translational and/or stretching modes. Meanwhile, these results are quite different from the case of CO2 dissociation, in which the antisymmetric stretching mode is the key to increasing the dissociation rate. Accordingly, we anticipate that this work may contribute to the future development in controlling the particular molecular vibrational mode for improving catalytic reactions.
The authors thank Prof. Junji Nakamura, Prof. Takahiro Kondo, and Dr Jiamei Quan for their valuable discussions. The present work was supported by the Advanced Catalytic Transformation Program for Carbon utilization (ACT-C) (Grant No. JPMJCR12YU) of the Japan Science and Technology Agency (JST), and partly supported by Grants-in Aid for Scientific Research on Innovative Areas 3D Active-Site Science (No. 26105010 and No. 26105011) from the Japan Society for the Promotion of Science (JSPS) and the Elements Strategy Initiative for Catalysts and Batteries (ESICB) supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan (MEXT). The numerical calculations were performed using the computer resources at the Institute for Solid State Physics (ISSP), Univ. of Tokyo, and the HPCI systems provided by Nagoya Univ., Univ. of Tokyo, and Tohoku Univ. through the HPCI System Research Project (Project ID: hp130112, hp140166, and hp150201).
References
- S. Kattel, P. J. Ramrez, J. G. Chen, J. A. Rodriguez and P. Liu, Science, 2017, 355, 1296–1299 CrossRef CAS PubMed.
- S. Kuld, M. Thorhauge, H. Falsig, C. F. Elkjær, S. Helveg, I. Chorkendorff and J. Sehested, Science, 2016, 352, 969–974 CrossRef CAS PubMed.
- M. Behrens, F. Studt, I. Kasatkin, S. Kühl, M. Hävecker, F. Abild-Pedersen, S. Zander, F. Girgsdies, P. Kurr, B.-L. Kniep, M. Tovar, R. W. Fischer, J. K. Nørskov and R. Schlögl, Science, 2012, 336, 893–897 CrossRef CAS PubMed.
- L. C. Grabow and M. Mavrikakis, ACS Catal., 2011, 1, 365–384 CrossRef CAS.
- T. Fujitani, I. Nakamura, T. Uchijima and J. Nakamura, Surf. Sci., 1997, 383, 285–298 CrossRef CAS.
- G. Wang, Y. Morikawa, T. Matsumoto and J. Nakamura, J. Phys. Chem. B, 2006, 110, 9–11 CrossRef CAS PubMed.
- H. Nakano, I. Nakamura, T. Fujitani and J. Nakamura, J. Phys. Chem. B, 2001, 105, 1355–1365 CrossRef CAS.
- H. Nishimura, T. Yatsu, T. Fujitani, T. Uchijima and J. Nakamura, J. Mol. Catal. A: Chem., 2000, 155, 3–11 CrossRef CAS.
- I. Nakamura, H. Nakano, T. Fujitani, T. Uchijima and J. Nakamura, J. Vac. Sci. Technol., A, 1999, 17, 1592–1595 Search PubMed.
- W. Lin, K. M. Stocker and G. C. Schatz, J. Am. Chem. Soc., 2017, 139, 4663–4666 CrossRef CAS PubMed.
- J. Quan, T. Kondo, G. Wang and J. Nakamura, Angew. Chem., Int. Ed., 2017, 56, 3496–3500 CrossRef CAS PubMed.
- Y. Morikawa, K. Iwata, J. Nakamura, T. Fujitani and K. Terakura, Chem. Phys. Lett., 1999, 304, 91–97 CrossRef CAS.
- F. Nattino, D. Migliorini, M. Bonfanti and G.-J. Kroes, J. Chem. Phys., 2016, 144, 044702 CrossRef PubMed.
- M. Dion, H. Rydberg, E. Schröder, D. C. Langreth and B. I. Lundqvist, Phys. Rev. Lett., 2004, 92, 246401 CrossRef CAS PubMed.
- J. Klimeš, D. R. Bowler and A. Michaelides, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 195131 CrossRef.
- I. Hamada, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 121103 CrossRef.
- Y. Hamamoto, I. Hamada, K. Inagaki and Y. Morikawa, Phys. Rev. B, 2016, 93, 245440 CrossRef.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- T. Shimanouchi, Tables of Molecular Vibrational Frequencies, Consolidated Volume 1, National Standard Reference Data System (NSRDS), Tokyo, Japan, 1972 Search PubMed.
- W. B. Person and G. Zerbi, Vibrational intensities in infrared and Raman spectroscopy, Elsevier Scientific Pub. Co., Amsterdam, Netherland, 1982 Search PubMed.
- B. Jiang and H. Guo, J. Chem. Phys., 2013, 138, 234104 CrossRef PubMed.
- B. Jiang and H. Guo, J. Chem. Phys., 2016, 144, 091101 CrossRef PubMed.
- T. Yamanaka, Phys. Chem. Chem. Phys., 2008, 10, 5429–5435 RSC.
- K. Nakao, S. Ito, K. Tomishige and K. Kunimori, Catal. Today, 2006, 111, 316–321 CrossRef CAS.
- K. Nakao, S. Ito, K. Tomishige and K. Kunimori, J. Phys. Chem. B, 2005, 109, 17553–17559 CrossRef CAS PubMed.
- K. Kimura, Y. Ohno and T. Matsushima, Surf. Sci., 1999, 429, L455–L461 CrossRef CAS.
- H. Uetsuka, K. Watanabe, H. Ohnuma and K. Kunimori, Surf. Sci., 1997, 377, 765–769 CrossRef.
- H. Uetsuka, K. Watanabe and K. Kunimori, Surf. Sci., 1996, 363, 73–78 CrossRef CAS.
- T. Engel and G. Ertl, J. Chem. Phys., 1978, 69, 1267–1281 CrossRef CAS.
- X. Zhou, B. Kolb, X. Luo, H. Guo and B. Jiang, J. Phys. Chem. C, 2017, 121, 5594–5602 CrossRef CAS.
- A. Alavi, P. Hu, T. Deutsch, P. L. Silvestrelli and J. Hutter, Phys. Rev. Lett., 1998, 80, 3650–3653 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional information. See DOI: 10.1039/c7cc03707d |
| This journal is © The Royal Society of Chemistry 2017 |