Breathing-induced new phase transition in an MIL-53(Al)–NH2 metal–organic framework under high methane pressures†
Abstract
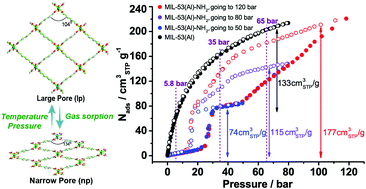
The high pressure methane sorption tests on a flexible metal–organic framework [MIL-53(Al)–NH2] reveal a new phase transition to a large pore (lp) phase above 45 bar. The mixed-ligand strategy and pressure cycling tests are suggested to reduce the pressure requirement of such a phase transition for applications in natural gas storage.


 Please wait while we load your content...
Please wait while we load your content...