Autonomously propelled microscavengers for precious metal recovery†
Abstract
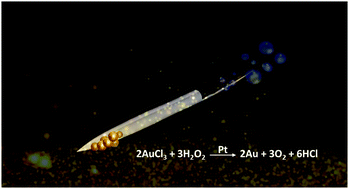
We report biogenic micromotor design consisting of porous chalky elongated tubes (∼60 μm length) coated with Fe–Pt for dual functionality i.e. metallic gold formation and rapid isolation. These autonomously propelled scavengers once introduced in the reaction environment, showed rapid bubble-propulsion followed by high-purity separation of the visually-distinguishable gold metal particles (yellow in colour) from the reaction mixture. The concept presented here has excellent potential towards environmentally sustainable metal recovery, micron-level metal/mineral particulate extraction, electronic waste treatment and similar redox product separation among others.


 Please wait while we load your content...
Please wait while we load your content...