Unique site-selectivity control in asymmetric Michael addition of azlactone to alkenyl dienyl ketones enabled by P-spiro chiral iminophosphorane catalysis†
Abstract
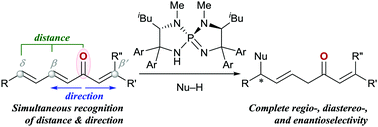
A highly regio-, diastereo-, and enantioselective 1,6-addition of azlactone to various alkenyl dienyl ketones has been achieved under P-spiro chiral triaminoiminophosphorane catalysis. This site-selectivity control relies on the distinct ability of the conjugate acids of iminophosphoranes, aminophosphonium ions, to precisely differentiate between the three electrophilic reaction sites of alkenyl dienyl ketones, which is further demonstrated by the discriminative asymmetric 1,6-addition of azlactone to dienyl ketones, leaving the coexisting alkenyl ketone intact.
- This article is part of the themed collection: Site-selective molecular transformations


 Please wait while we load your content...
Please wait while we load your content...