Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Glucose selective bis-boronic acid click-fluor†‡
Wenlei
Zhai
a,
Louise
Male
b and
John S.
Fossey
 *a
*a
aSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, West Midlands, B15 2TT, UK. E-mail: J.s.fossey@bham.ac.uk
bX-Ray Crystallography Facility, School of Chemistry, University of Birmingham, Edgbaston, Birmingham, West Midlands, UK
First published on 1st December 2016
Abstract
Four novel bis-boronic acid compounds were synthesised via copper catalysed azide–alkyne cycloaddition (CuAAC) reactions. Glucose selectivity was observed for a particular structural motif. Moreover, a new glucose selective fluorescent sensor was designed and synthesised as a result.
In the past few decades, synthetic probes have shown significant promise for real-time and accurate detection of biomolecules.1 Much attention has been devoted to boronic acid derivatives for saccharide detection.2 Although boronic acid-mediated saccharide sensing showed encouraging results, a lack of selectivity for higher order saccharides needs to be addressed, and remains challenging. Phenylboronic acid derivatives have greater affinity for fructose over glucose, under physiological conditions.3 For the purpose of increasing the binding affinity to glucose, and other saccharides, a more sophisticated receptor structure is required.
James et al. were the first to report that two appropriately positioned boronic acids could modulate selectivity by two-point binding interactions with glucose (sensor 1, Fig. 1).4 Further studies conducted by James et al. demonstrated that design of the spacer unit between the borons, of a bis-boronic acid, was crucial for glucose recognition (sensor 2, Fig. 1).5 Drueckhammer et al. showed that the distance between two p-tolylboronic acids could be optimised, for glucose selectivity, through a computational study, and a rigid four-fused ring scaffold was produced (sensor 3, Fig. 1).6 Thus, appropriate positioning of boronic acids can give glucose selectivity. However, application of a universally simple methodology to selective saccharide receptor design remains a challenge.7
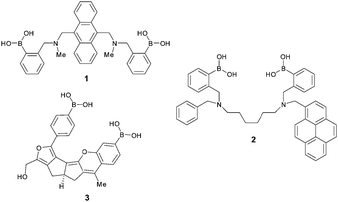 | ||
| Fig. 1 Chemical structures of reported glucose-selective fluorescent sensors developed by Shinkai et al. (1),4a James et al. (2)5a and Drueckhammer et al. (3).6 | ||
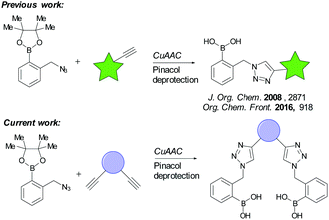
Copper catalysed azide–alkyne cycloaddition (CuAAC) reaction, often referred to as the “click reaction,”8 has been employed to synthesise novel boronic acid derivatives for various applications. In order to explore the advantages of the CuAAC reaction in the area of saccharide sensing,9 Scrafton et al. employed the CuAAC reaction for a five-step synthesis of a boronic acid-based sensor molecule (Scheme 1).10 The term “click-fluor” was used to refer to this class of molecular sensors. Recently, the scope has been elaborated to include incorporation of fluorophores.11 Although these studies demonstrated the possibility of rapid synthesis of boronic acids via the CuAAC reaction, the reported mono-boronic acids cannot serve as glucose-selective sensors due to their relatively weak glucose binding. In order to engineer selectivity, CuAAC may be employed to construct bis-boronic acids. Wang and co-workers reported two triazole-linked bis-α-amidoboronic acids.12 Their result showed significantly enhanced binding affinity for oligosaccharides. Zhao et al. also designed and synthesised three bis-boronic acid sensors through triazole formation.13 However, in their studies, the receptors preferentially recognise D-fructose over D-glucose.
In this report, three bis-boronic acid molecules were designed such that the CuAAC reaction may be used to rapidly construct a series. Their synthesis is studied and saccharide binding evaluated, for the purpose of developing a novel multi-boronic acid “click” platform. As such, we built upon the knowledge that glucose selectivity may be achieved by correctly spacing two boronic acids in one molecule, to demonstrate “click-compatibility” for selectivity, and pave the way for future exploration in higher order saccharide sensor design.
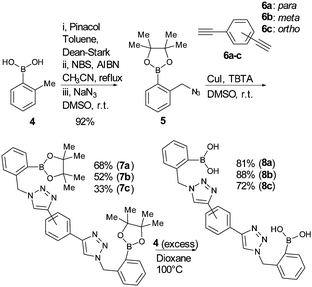
Three regio-isomeric bis-boronic acids (8a–c), akin to the mono-boronic acid click-fluors already reported, were designed and their synthesis embarked upon (see Scheme 2). Following pinacol protection of commercially available o-tolylboronic acid, organic azide 5 was synthesised on a 10 gram scale, by bromination and displacement of bromide by azide, according to literature procedures (92% yield of compound 5).11 Three bis-alkynes were required; fortunately 1,3- and 1,4-diethynylbenzene are commercially available and were used as purchased. 1,2-Diethynylbenzene was readily synthesised from 1,2-dibromobenzene via a palladium-catalysed Sonogashira coupling and TMS removal following literature procedures.14 Initially, the CuAAC reaction was conducted as per our previous reports, but poor yields of the target bis-boronic esters (7a–c), as a result of unwanted side reactions, plagued our experiments.15 For example, during the synthesis of compound 7b, oxidation and deborylation reactions occurred on one or both of the boronic esters (confirmed by mass spectrometry). Therefore, the conditions of the CuAAC reaction were further modified. More mild conditions, use of TBTA as a ligand for copper and lessening of catalyst loading helped improve the reaction outcomes and minimize (copper-catalysed) de-borylation.16 Thus, the yields of these three key intermediates were improved from 32% to 68% (7a), 21% to 52% (7b), and 18% to 33% (7c), respectively. Next, pinacol was removed by addition of compound 4 under acidic conditions; note that this gives a by-product, pinacol protected-4, which may be (and was) used in further syntheses.13a Bis-boronic acids 8a–c were obtained after trituration and flash chromatography in 72–88% isolated yield.
The bis-pinacol esters, 7a–c, are crystalline solids, and crystals suitable for single crystal XRD structure determination were grown from mixtures of hexane and ethyl acetate. From the obtained structures presented in Fig. 2, the distance between the two boron atoms was measured as para7a B(1)⋯B(1)i = 14.787 (3) Å; 7bmeta B(1)⋯B(2) = 14.101 (4) Å; and ortho7c B(1)⋯B(1)ii = 7.619 (3) Å for each compound.17 The distance in 7c is obviously reduced compared to that in 7a.
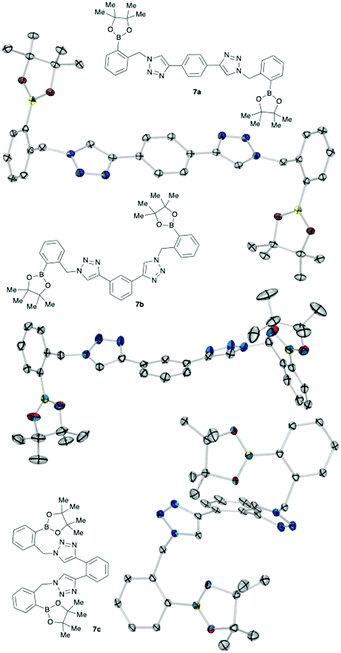 | ||
| Fig. 2 Chemical and X-ray structures of key intermediates 7a–c (ORTEP ellipsoids 30% probability rendered using Ortep III for Windows and PovRay). Hydrogen atoms have been omitted. In 7a and 7c the molecules are located on a symmetry element such that only half in each case are crystallographically unique. In 7c, part of the boronic ester group is disordered over two positions, with only the major part being shown here.17 | ||
To determine the saccharide binding capability of the synthesised bis-boronic acids, isothermal titration calorimetry (ITC) was employed. ITC is an effective method to study the binding affinity between small molecules and large biomolecules like proteins and DNA.18 Moreover, there are studies of using ITC to determine the binding strength between lectins and saccharide,19 as well as boronic acid containing molecules with saccharide.11,20 In our experiment, measurements were carried out in pH 8.21 PBS buffer with up to 20% DMSO, depending on the solubility of the tested compounds.
According to the ITC results, compound 8a behaves like most mono-boronic acid derivatives, showing higher binding affinity for fructose than glucose. As shown in Table 1, the recorded binding constant between 8a and fructose is 1.90 × 103 ± 280 M−1, which is similar to that of mono-boronic acid.11
| 8a | 8b | 8c | |
|---|---|---|---|
| Binding site (fructose) | 1.89 ± 0.280 | 0.48 ± 0.005 | 1.30 ± 0.046 |
| Binding constant (M−1) (fructose) | 1.90 × 103 ± 280 | 1.30 × 105 ± 1.10 × 104 | 2.95 × 103 ± 183 |
| Binding site (glucose) | N/A | 0.45 ± 0.081 | 1.33 ± 0.101 |
| Binding constant (M−1) (fructose) | N/A | 5.03 × 103 ± 479 | 6.19 × 103 ± 731 |
However, attempts to determine the binding constant between 8a and glucose failed due to such a weak interaction (see the ESI,‡ Fig. S2). Compared with compound 8a, the glucose binding affinity of compound 8b is improved. Surprisingly, the binding constant between 8b and fructose is extremely high. Indeed, this superior fructose selectivity is noteworthy and further investigations are planned to better understand this observation. For both 8b and 8c, it takes at least 20 minutes to reach the equilibrium for each addition of glucose. On the other hand, each fructose addition equilibrates within 5 minutes under the same conditions. Perhaps, the glucose binding process requires the conformational change of analyte or receptor to facilitate the optimal interaction.
As presented in Fig. 3, compound 8c showed good binding affinities to both fructose and glucose. The binding strength between 8c and glucose is more than twice that of fructose. Therefore, the ITC data reveal that compound 8c is a glucose selective receptor. The binding constants of compound 8a–c with glucose increase across meta, para and ortho series respectively, which again demonstrates that the distance between the two boronic acid groups is critical for glucose recognition.
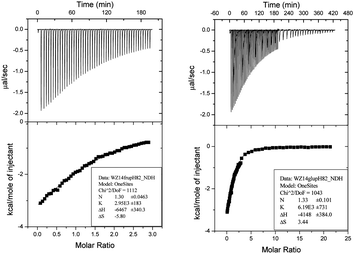 | ||
| Fig. 3 ITC study of compound 8c binding with fructose (left) and glucose (right) in pH 8.21 PBS buffer with 20% DMSO. | ||
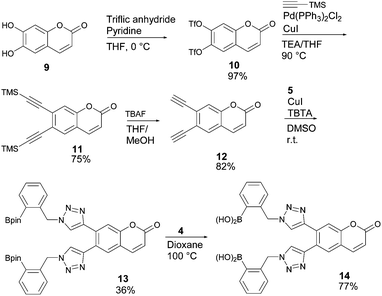
Encouraged by the positive result from the ITC experiment, we decided to combine this newly developed receptor architecture with a fluorophore, in order to construct a glucose-selective fluorescence sensor. Starting from commercially available 6,7-dihydroxycoumarin (9), bis-alkyne 12 was synthesised by converting the hydroxyl groups into triflates (10), followed by Sonogashira coupling and TMS deprotection using TBAF (Scheme 3).21 The CuAAC reaction and pinacol deprotection were performed under the same conditions as earlier.
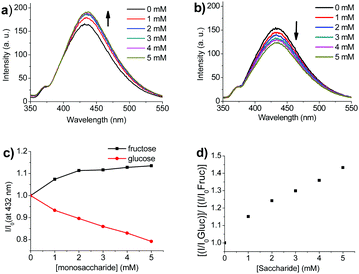
In Fig. 4, the fluorescence response of sensor 14 towards fructose and glucose is shown, respectively. It was observed that the fluorescence intensity was quite weak for sensor 14, as the Raman scattering signal of the excitation source was also recorded on the spectrum. Upon addition of 5 mM fructose, the fluorescence signal was slightly enhanced (I/I0 = 1.13, see Fig. 4a), which was similar to what we have observed with mono-boronic acid sensor binding with fructose in our previous study.11
However, the fluorescence intensity gradually decreased upon addition of 5 mM glucose (I/I0 = 0.79, see Fig. 4b and c). Presumably, the different fluorescence responses towards fructose and glucose were caused by the differing binding modes. Meanwhile, it took no less than 30 minutes for the fluorescence signal to be stabilised after adding glucose, which agrees with the slow binding process in our ITC studies.
Three regioisomeric bis-boronic acids were synthesised using the CuAAC reaction. The binding constants of the synthesised compounds with fructose and glucose were measured by ITC experiment, respectively. It was found that selectivity for glucose binding is modulated by the distance between the two boronic acid groups. To our delight, compound 8c presented higher binding affinity towards glucose over fructose. Moreover, a fluorescent receptor, 14, was synthesised showing divergent properties upon interaction with glucose versus fructose. It was shown that compound 14 can serve as an “on–off” fluorescence sensor for selective glucose detection. More studies are required to better understand the fluorescence modulation mechanism, which is an ongoing work in our laboratory. We showed the utility of combining different functional components using the CuAAC reaction to construct selective molecular receptors for more challenging targets.
WZ and JSF thank The Catalysis and Sensing for our Environment (CASE) group for networking opportunities.22 China Scholarship Council (CSC) and the University of Birmingham are also thanked for providing full-fee studentship support to WZ. JSF thanks the University of Birmingham for support, the Royal Society for an Industrial Fellowship (6955) and the EPSRC for funding (EP/J003220/1).
Notes and references
- A. P. de Silva, T. S. Moody and G. D. Wright, Analyst, 2009, 134, 2385–2393 RSC.
- (a) X. Sun and T. D. James, Chem. Rev., 2015, 115, 8001–8037 CrossRef CAS PubMed; (b) W. Zhai, X. Sun, T. D. James and J. S. Fossey, Chem. – Asian J., 2015, 10, 1836–1848 CrossRef CAS PubMed; (c) X. Sun, W. Zhai, J. S. Fossey and T. D. James, Chem. Commun., 2016, 52, 3456–3469 RSC.
- (a) J. P. Lorand and J. O. Edwards, J. Org. Chem., 1959, 24, 769–774 CrossRef CAS; (b) S. Guo, J. Chen, B.-Y. Cai, W.-W. Chen, Y.-F. Li, X. Sun, G.-R. Chen, X.-P. He and T. D. James, Materials Chemistry Frontiers, 2017 10.1039/C6QM00158K.
- (a) T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Angew. Chem., Int. Ed., 1994, 33, 2207–2209 CrossRef; (b) T. D. James, K. R. A. S. Sandanayake, R. Iguchi and S. Shinkai, J. Am. Chem. Soc., 1995, 117, 8982–8987 CrossRef CAS.
- (a) S. Arimori, M. L. Bell, C. S. Oh, K. A. Frimat and T. D. James, Chem. Commun., 2001, 1836–1837 RSC; (b) S. Arimori, M. L. Bell, C. S. Oh, K. A. Frimat and T. D. James, J. Chem. Soc., Perkin Trans. 1, 2002, 803–808 RSC.
- W. Yang, H. He and D. G. Drueckhammer, Angew. Chem., Int. Ed., 2001, 40, 1714–1718 CrossRef CAS.
- J. S. Fossey, F. D'Hooge, J. M. H. van den Elsen, M. P. Pereira Morais, S. I. Pascu, S. D. Bull, F. Marken, A. T. A. Jenkins, Y.-B. Jiang and T. D. James, Chem. Roc., 2012, 12, 464–478 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- C. Dai, Y. Cheng, J. Cui and B. Wang, Molecules, 2010, 15, 5768–5781 CrossRef CAS PubMed.
- D. K. Scrafton, J. E. Taylor, M. F. Mahon, J. S. Fossey and T. D. James, J. Org. Chem., 2008, 73, 2871–2874 CrossRef CAS PubMed.
- W. Zhai, B. M. Chapin, A. Yoshizawa, H.-C. Wang, S. A. Hodge, T. D. James, E. V. Anslyn and J. S. Fossey, Org. Chem. Front., 2016, 3, 918–928 RSC.
- S. Jin, C. Zhu, Y. Cheng, M. Li and B. Wang, Bioorg. Med. Chem., 2010, 18, 1449–1455 CrossRef CAS PubMed.
- (a) M. Shao and Y. Zhao, Tetrahedron Lett., 2010, 51, 2508–2511 CrossRef CAS; (b) K. Mulla, P. Dongare, N. Zhou, G. Chen, D. W. Thompson and Y. Zhao, Org. Biomol. Chem., 2011, 9, 1332–1336 RSC.
- Q. Zhou, P. J. Carroll and T. M. Swager, J. Org. Chem., 1994, 59, 1294–1301 CrossRef CAS.
- S. Jin, G. Choudhary, Y. Cheng, C. Dai, M. Li and B. Wang, Chem. Commun., 2009, 5251–5253 RSC.
- T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Org. Lett., 2004, 6, 2853–2855 CrossRef CAS PubMed.
- See ESI for atom labels and further details and corresponding CIF files. CCDC deposition numbers 1510897–1510899. (i) Symmetry code for the generation of equivalent atoms: −x, 1 − y, 2 − z; (ii) symmetry code for the generation of equivalent atoms: −x, y, 1/2 − z.
- M. W. Freyer and E. A. Lewis, Methods in Cell Biology, Academic Press, 2008, vol. 84, pp. 79–113 Search PubMed.
- T. K. Dam and C. F. Brewer, Chem. Rev., 2002, 102, 387–430 CrossRef CAS PubMed.
- (a) Ö. Torun, F. C. Dudak, D. Baş, U. Tamer and İ. H. Boyacı, Sens. Actuators, B, 2009, 140, 597–602 CrossRef; (b) M. S. Melicher, J. Chu, A. S. Walker, S. J. Miller, R. H. G. Baxter and A. Schepartz, Org. Lett., 2013, 15, 5048–5051 CrossRef CAS PubMed.
- Z. Zhou and C. J. Fahrni, J. Am. Chem. Soc., 2004, 126, 8862–8863 CrossRef CAS PubMed.
- (a) D. T. Payne, J. S. Fossey and R. B. P. Elmes, Supramol. Chem., 2016, 28, 921–931 CrossRef CAS; (b) J. S. Fossey and W. D. G. Brittain, Org. Chem. Front., 2015, 2, 101–105 RSC.
Footnotes |
| † Dedicated to the 60th Birthday of Tony Czarnik. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1510897–1510899. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc08534b |
| This journal is © The Royal Society of Chemistry 2017 |