Introducing multiple bio-functional groups on the poly(ether sulfone) membrane substrate to fabricate an effective antithrombotic bio-interface†
Abstract
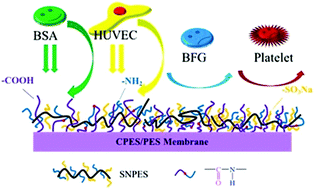
It has been widely recognized that functional groups on biomaterial surfaces play important roles in blood compatibility. To construct an effective antithrombotic bio-interface onto the poly(ether sulfone) (PES) membrane surface, bio-functional groups of sodium carboxylic (–COONa), sodium sulfonic (–SO3Na) and amino (–NH2) groups were introduced onto the PES membrane surface in three steps: the synthesis of PES with carboxylic (–COOH) groups (CPES) and water-soluble PES with sodium sulfonic (–SO3Na) groups and amino (–NH2) groups (SNPES); the introduction of carboxylic groups onto the PES membrane by blending CPES with PES; and the grafting of SNPES onto CPES/PES membranes via the coupling of amino groups and carboxyl groups. The physical/chemical properties and bioactivities were dependent on the proportions of the additives. After introducing bio-functional groups, the excellent hemocompatibility of the modified membranes was confirmed by the inhibited platelet adhesion and activation, prolonged clotting times, suppressed blood-related complement and leukocyte-related complement receptor activations. Furthermore, cell tests indicated that the modified membranes showed better cytocompatibility in endothelial cell proliferation than the pristine PES membrane due to the synergistic promotion of the functional groups. To sum up, these results suggested that modified membranes present great potential in fields using blood-contacting materials, such as hemodialysis and surface endothelialization.

 Please wait while we load your content...
Please wait while we load your content...