A simple technique to design microfluidic devices for system integration
Abstract
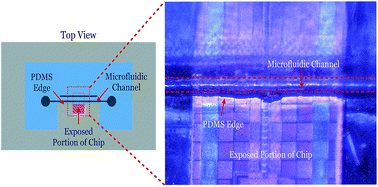
We present a robust method to fabricate a polydimethylsiloxane (PDMS) microfluidic device with critical channel features located near the periphery. The fabricated device has a window cutout, allowing close placement and integration of the channel with solid state circuits and devices. The flow characteristics of the fabricated device are shown to closely follow that of theoretical behavior, making it an appropriate alternative to a monolithic, cast-molded device. As an illustration of the use of this method, a novel integrated microfluidic-electronic system is demonstrated with the microfluidic channel bonded directly onto the surface of an integrated circuit while leaving access to probe pads on the chip's surface. This fabrication process has important implications in furthering the development of next generation microfluidic-based lab on chip systems.


 Please wait while we load your content...
Please wait while we load your content...